As temperature increases the rate of a reaction does this.
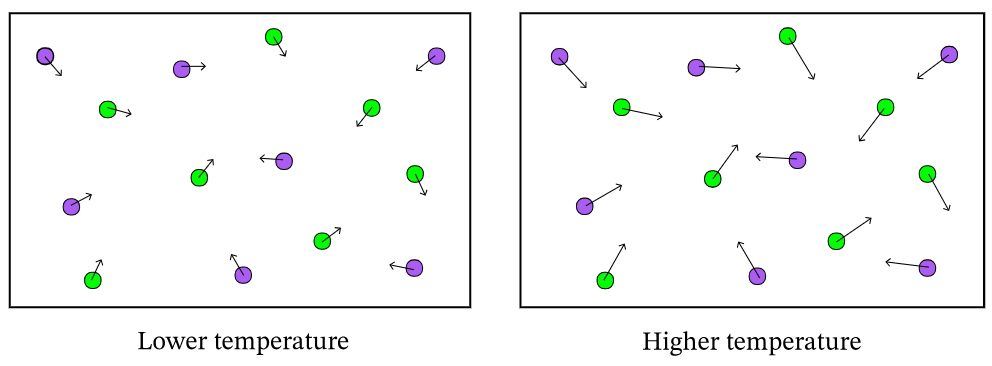
What is increases?
The number of moles H2 in 20 grams H2.
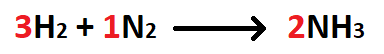
What is 10 moles H2?
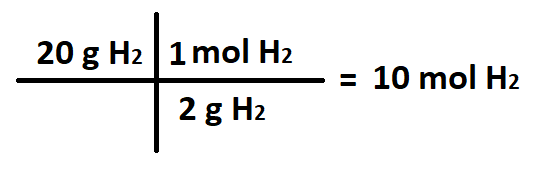
What tool do you use to find a molar mass ratio?
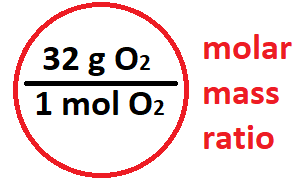
What is the Periodic Table?
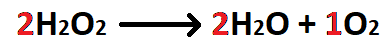
A student wants to convert 2 moles of H2O2 into grams. What does the X stand for in the conversion below?
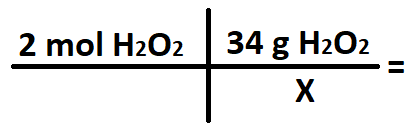
What is 1 mol H2O2?
The section of this graph showing the fastest rate.
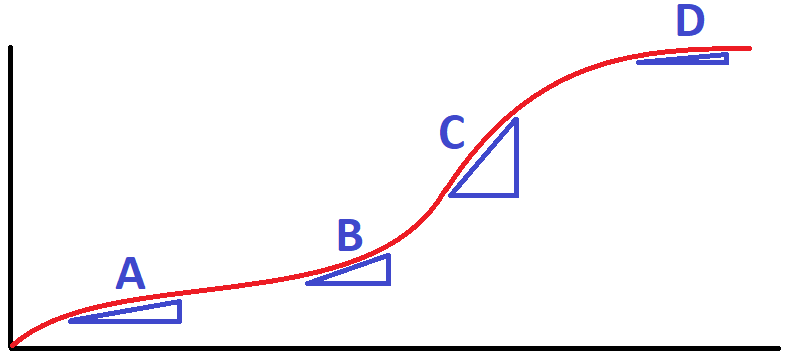
What is section C?

The reason that a higher concentration increases the rate of a reaction.
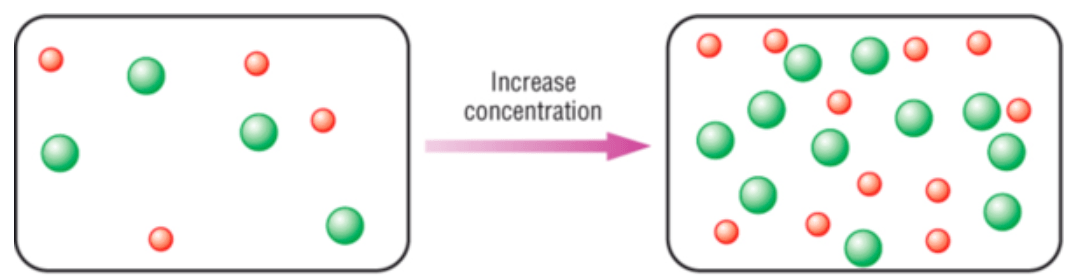
What is more frequent collisions?
The number of moles NH3 produced when 6 moles of H2 react with excess N2.
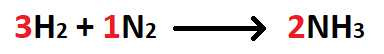
What is 4 moles NH3?
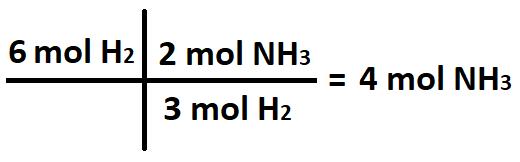
What tool do you use to find a molar ratio?
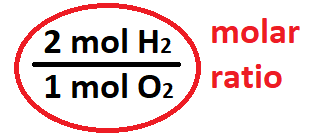
What is a balanced chemical equation?
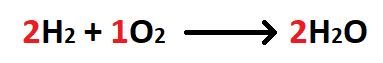
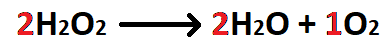
A student wants to convert 6 moles of H2O2 into moles of O2. What does the X stand for in the conversion below?
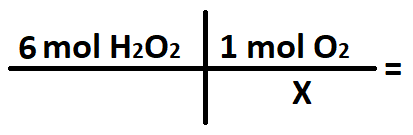
What is 2 mol H2O2?
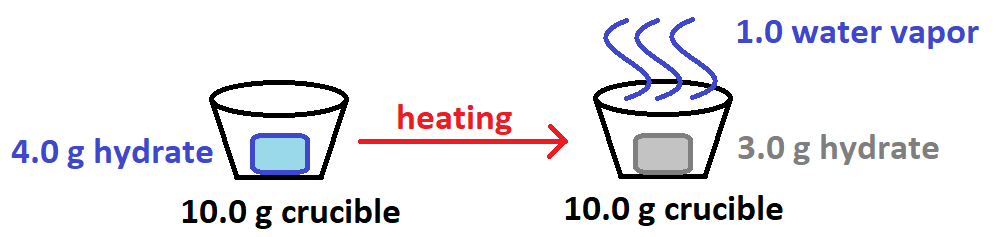
A student heats a sample of a hydrate and collects the following data:
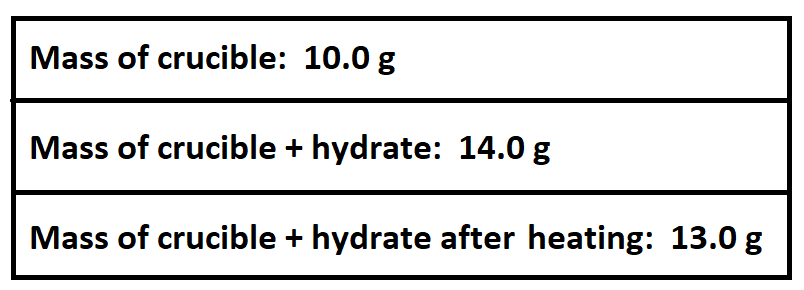
What mass of water was released during heating?
What is 1.0 gram water?
This increases the rate of a reaction by lowering the activation energy.
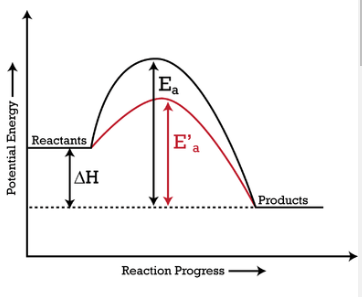
What is a catalyst?
The number of moles NH3 produced when 28 grams of N2 react with excess H2.
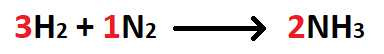
What is 2 moles NH3?
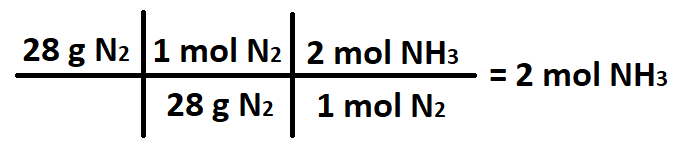
The molar mass ratio of H2O.
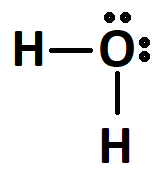
What is 18 g / 1 mol?
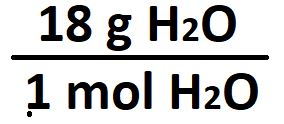
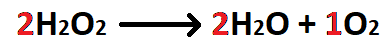
A student wants to convert 20 grams of H2O2 into moles of O2. What does the X stand for in the conversion below?
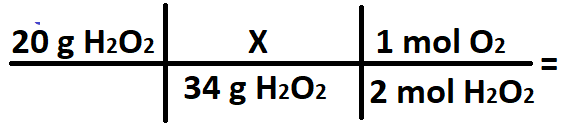
What is 1 mol H2O2?
The three principles of collision theory.

What are that particles...
1: must collide
2: with enough energy to break bonds
3: and in the correct orientation
The following diagram demonstrates an increase in _________ on the rate of a reaction.
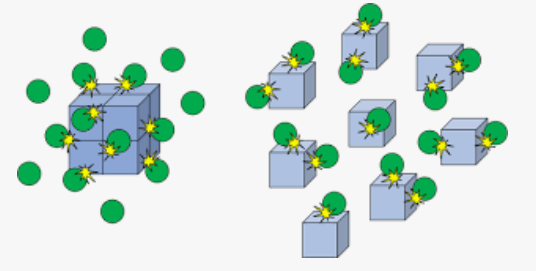
What is surface area?
The number of grams H2 used when 8 moles of NH3 are produced.
What is 24 grams H2?
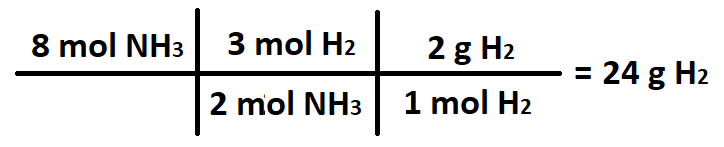
The molar ratio between hydrogen and oxygen.
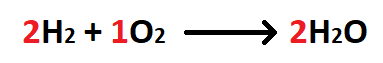
What is 2 mol H2: 1 mol O2?
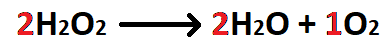
A student wants to convert 20 grams of H2O2 into grams of O2. What does the X stand for in the conversion below?
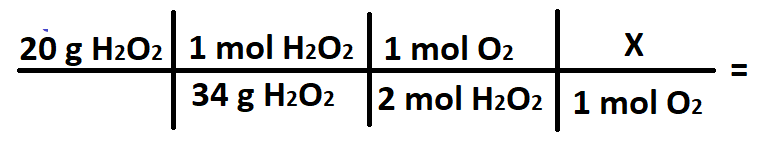
What is 32 g O2?
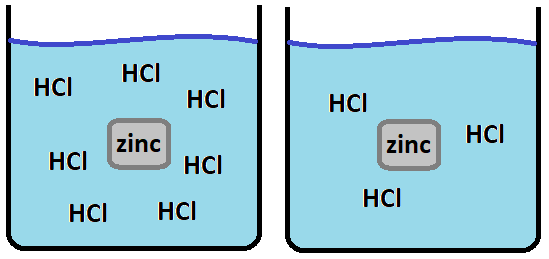
The reason that a piece of zinc metal dissolves faster in a concentrated acid than a dilute acid.
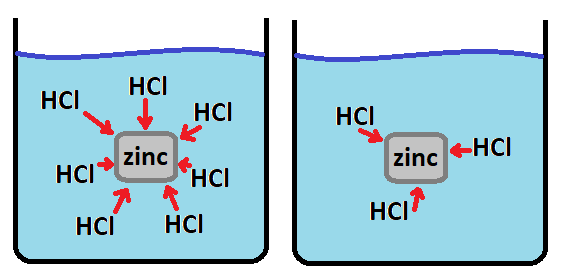
What is more frequent collisions?
The rate of this reaction for the first 6 seconds.
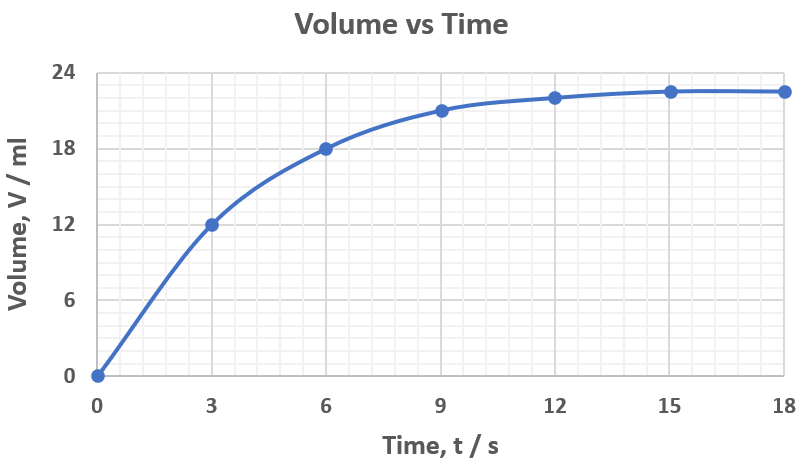
What is 3 ml/s?
The number of grams NH3 produced when 3 grams of H2 react with excess N2.
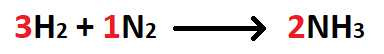
What is 17 grams NH3?
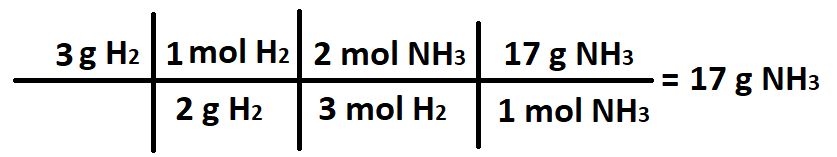
The ratio of grams H2 to grams O2.
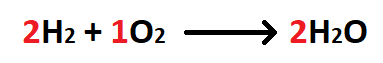
What is 1 gram H2 : 8 grams O2?
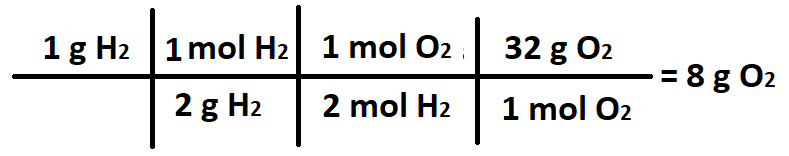
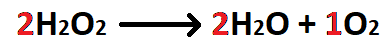
A student wants to convert 20 grams of H2O2 into grams of O2. What does the X stand for in the conversion below?
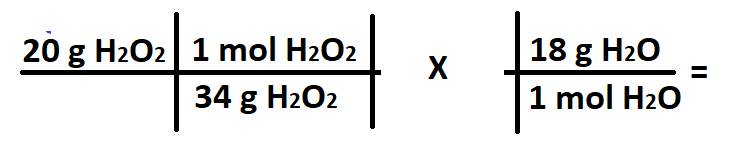
What is 2 mol H2O : 2 mol H2O2?
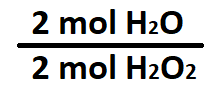
The reason(s) why one beaker has a faster reaction than the others.
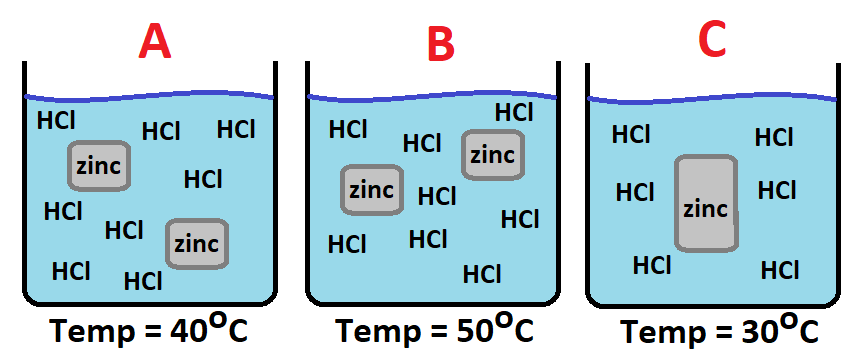
Beaker B has the fastest reaction because it has the highest temperature, resulting in more frequent and harder collisions.
Beaker B also has a high concentration of acid and a large surface area of zinc.
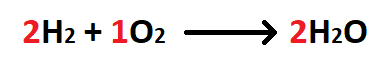
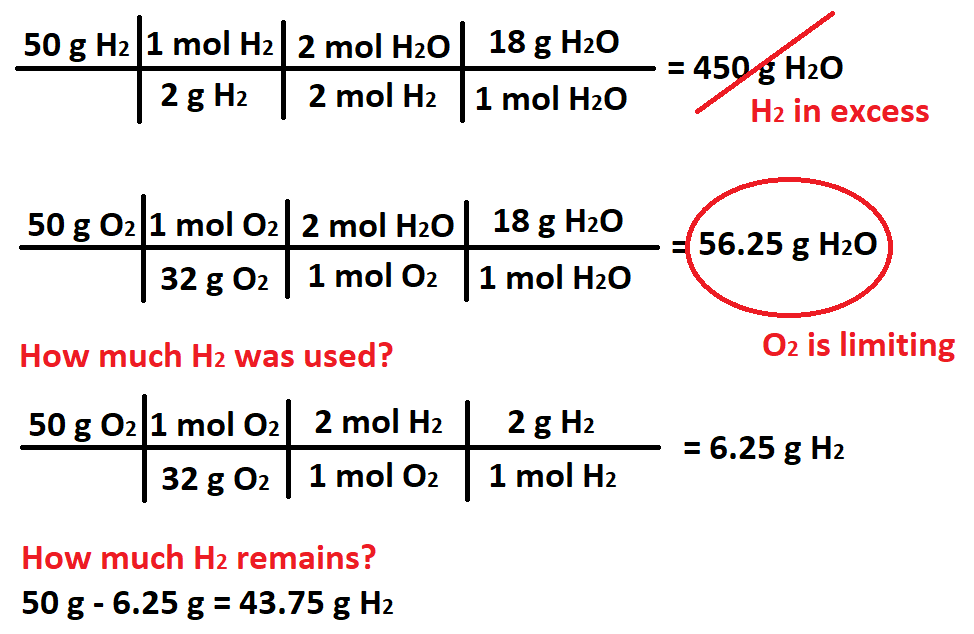
50 g H2 is mixed with 50 g O2. When the reaction is complete, how many grams of the excess reactant remain?
What is 43.75 g H2?