The change in the concentration of reactants during a chemical reaction (increases or decreases)
What is decreases?
Letter A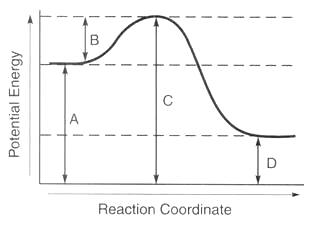
What is potential energy of the reactants?
Collision Theory's rules of engagement
What is particles have to collide with correct orientation and sufficient energy to cause a reaction?
The overall order of the reaction with a rate law of r=[O2]2[H2S][Al]2
What is five
(In general) the relationship between the concentration of reactants and the speed of a reaction.
Formula for the rate of reaction:
rate = ^[reactants] / ^time
Letter C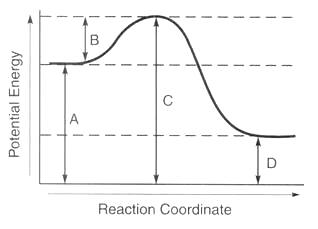
What is the activated complex?
Explanation of why a powdered reactant will react faster than a lump reactant?
What is greater surface area?
The order of the reaction based on this diagram
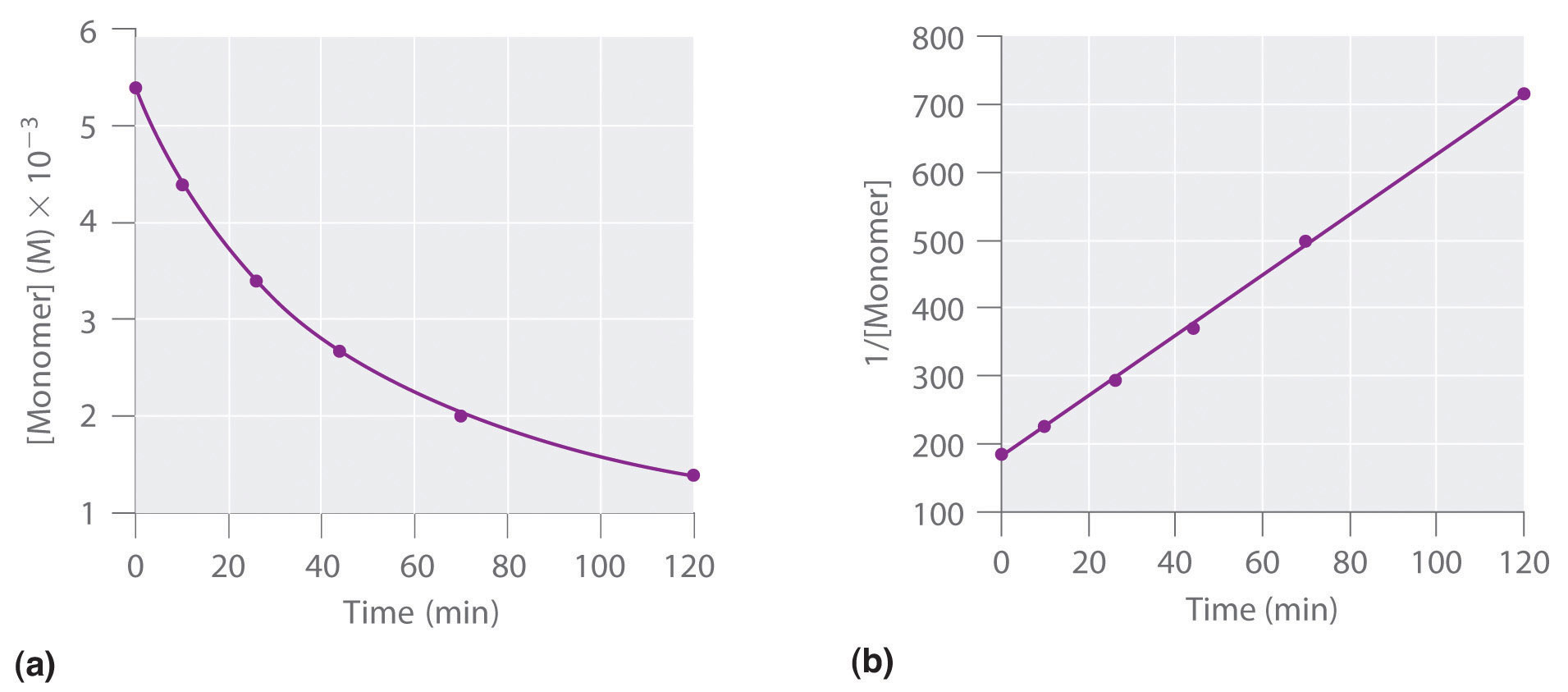
What is second order
These species are both made and consumed during a reaction mechanism
What is an intermediate?
As temperature is increased, particles have more
What is energy
Letter B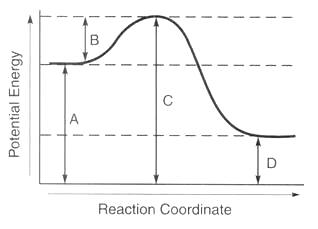
What is activation energy?
These two pieces make up the units for the rate constant
What are molarity(concentration) and time?
Despite not being zero my half life does not change with concentration
What is a first order reaction?
What is faster particles causes greater collisions?
How a catalyst speeds up a reaction.
What is lowers the activation energy?
The unit for the x axis. (The reaction coordinate).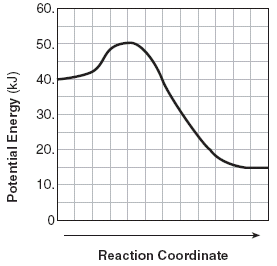
What is seconds (time)?
The transitionary substance that is created when the bonds are breaking or forming.
What is an activated complex?
My reaction order is described by this equation
A] = [A]0 − kt
What is a zero order reaction?
What is kinetics?
N2 + 3F2 -> 2NF3
F2 decreases at ______ the rate which NF3 increases
What is 3/2
The activation energy required (kJ).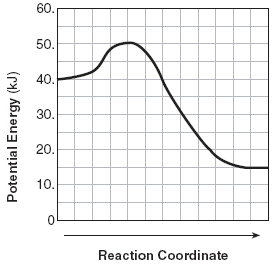
What is 10kJ?
CF4 + 2Br2 -> CBr4 + 2F2
The substance which increases at the fastest rate.
What is F2 (Fluorine)?
The factor reaction rate increases for a reaction described by r= k[O3][PS5]2 if the concentration of O3 triples and PS5 doubles.
What is twelve?
2AlBr3 + 3K2SO4 -> 6KBr + Al2(SO4)3
KBr increases/decreases at a rate of ____ times slower/faster than AlBr3 increases/decreases
Hint: This answer has 4 parts
What is increases, 3 times, faster, decreases?