g, mol, pariticles
How many inches is 27.4 feet?
12 in = 1 ft 1 in = 2.54 cm
27.4 ft xx (12 Inches)/(1 ft) = 328.8 Inches
If you have 20 moles of lithium, how many atoms is that?
20 mol xx(6.02e23 "atoms")/(1 mol)= 1.204 e 25 "atoms"
1.204e25 = 1.204 x 10^25
What is the molar mass of caffeine?
C8H10N4O2
UNITS
194.19 g/mol
You need units!
4Fe + 3O2 --> 2Fe2O3
How many grams of oxygen will react with 10 g iron?
10 g Fe xx(1 mol Fe)/(55.85 g Fe)xx(3 mol O2)/(4 mol Fe)xx(32 g O2)/(1 mol O2)=4.3gO2
Teal
If your door is 678 centimeters (cm) long, how many feet is that?
How many inches is 27.4 feet?
12 in = 1 ft 1 in = 2.54 cm
678 cm xx (1 Inch)/(2.54 cm) xx (1 ft)/(12 Inches)= 22.2 ft
If you have 9.8x1025 formula units of Li2O, how many moles is that?
9.8e25FU xx(1 mol)/(6.02e23 FU) = 162.8 mol
What is the mole ratio between Fe and Fe2O3?
2Fe2O3 + 3C --> 4Fe + 3CO2
2 mol Fe2O3 = 4 mol Fe
CH4 + 2O2 --> CO2 + 2H2O
If 5 g of methane (CH4) reacts with excess oxygen. How many grams of carbon dioxide should we expect?
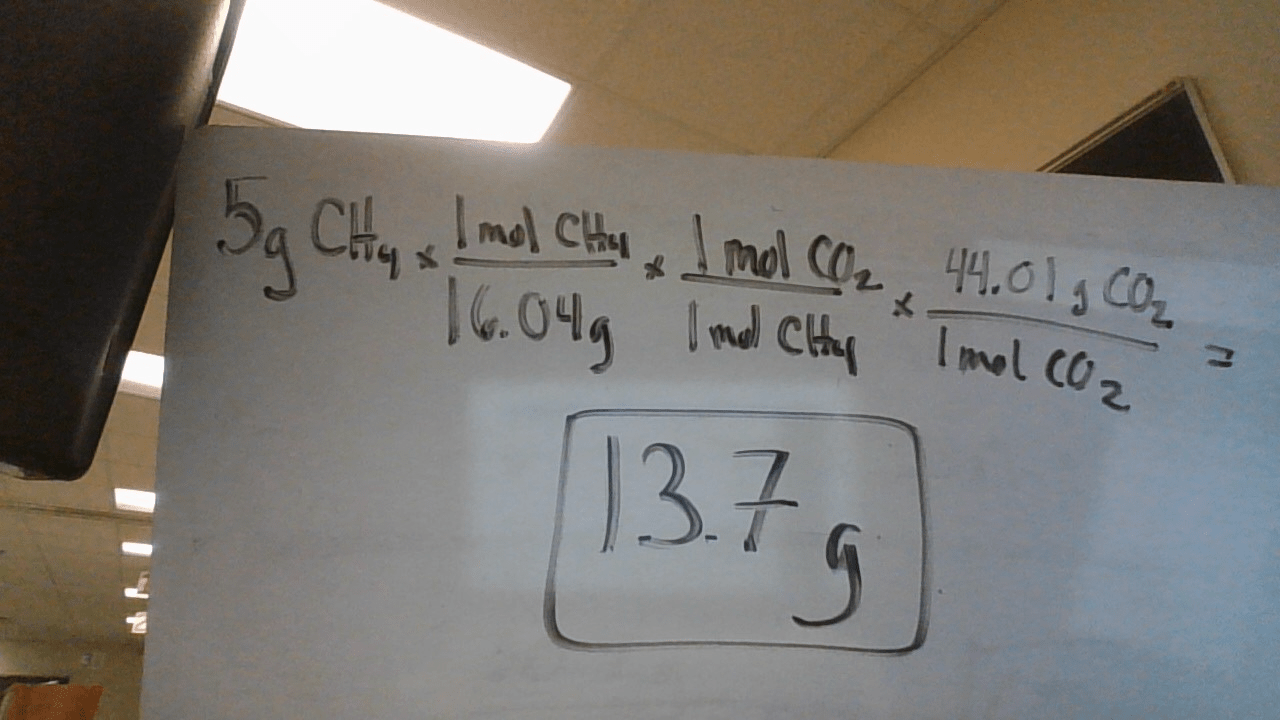
How tall is Mrs. O?
5' 7''
How many dollars is 697 quarters?
1 dollar = 4 quarters
697 quarters xx (1 dollar)/(4 quarters) = 174.25 dollars
500 grams of CO2 is how many moles?
500 g xx (1 mol CO2)/(44.01 g CO2)=11.36 mol
What is the molar mass of Ba(NO3)2?
Ba x 1 N x 2 O x 3
Use periodic table
261.34 g/mol
261 g Ba(NO3)2 = 1 mol Ba(NO3)2
2I2 + KIO3 + 6HCl --> 5ICl + KCl +3H2O
If 5.4 g of iodine reacts, what is the theoretical yield of ICl?
5.4 g I2 xx (1 mol I2)/(253.8 g I2) xx(5 mol ICl)/(2 mol I2) xx (162.35 gICl)/(1 mol ICl)= 8.6 g ICl
What color are Mrs. O's eyes?
blue
If Mrs. O watched 4.5 episodes of Spongebob on her treadmill, how many miles did she walk?
1 episode = 11 minutes
3 miles = 1 hr (her pace)
1 hr = 60 minutes
4.5 ep xx (11 min)/(1 ep)xx(1 hr)/(60 min)xx(3 mi)/(1 hr) = 2.475 mi
If you have 5.3 x 1022 atoms of Mg, how many grams is that?
5.3e22 "atoms" xx (1 mol)/(6.02e23 "atoms") xx (24.31 g)/(1 mol) = 2.1 g
What is the mole ratio needed if you know your starting amount of NaHCO3 and need to predict your ending amount of Na2SO4?
2NaHCO3(aq) + H2SO4(aq) --> Na2SO4(aq) + 2H2O(l) + 2CO2(g)
2 mol NaHCO3 = 1 mol Na2SO4
2NaCl --> 2Na + Cl2
You have 7 g of NaCl decomposing, what is the amount of Cl2 you will produce?
7 g NaCl xx(1 mol NaCl)/(58.44g NaCL)xx(1 mol Cl2)/(2 mol NaCl)xx(70.91 g Cl2)/(1 mol Cl2)= 4.25g Cl2
4.25 g Cl2
What is Mrs. O's favorite season?
Fall - When the air is crisp but not cold. Think pumpkin patch/apple orchard weather.
If you are 16.5 years old, how many seconds old are you?
1 yr = 365 days 1 day = 24 hours
1 hour = 60 minutes 1 minute = 60 seconds
16.5 yr xx (365 day)/(1 yr) xx (24 hr)/(1 day) xx (60 min)/(1 hr) xx (60 sec)/(1 min) = 5.2e8 sec
5.2e8 = 520344000 rounded
If you have 30 g of SO2, how many molecules is that?
30 g xx(1 mol)/(64.07 g)xx(6.02e23 "molecules")/(1 mol)=2.82e23"molecules"
2Fe2O3 + 3C --> 4Fe + 3CO2
If you have 68 moles Fe2O3, how many moles of Fe could you create?
68 mol Fe2O3 xx(4 mol Fe)/(2 mol Fe2O3) = 136 mol Fe
Pb(CH3COO)2 + H2S --> PbS + 2CH3COOH
If you create 27.3 g PbS, how many grams of CH3COOH do you also create?
27.3 g PbS xx(1mol PbS)/(239.27 gPbS)xx(2 mol CH3COOH)/(1 mol PbS)xx(60.06gCH3COOH)/(1 mol CH3COOH)=13.7 g CH3COOH
Has Mrs. O been sky diving, and did she live?
Mrs. O has not and will never sky dive.