Draw the lewis structure for a molecule of sulfur dioxide, SO2.
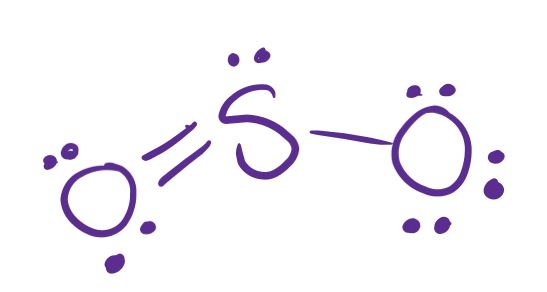
When the air bag inflates, the nitrogen gas is at a pressure of 1.30 atmospheres, a temperature of 301 K, and has a volume of 40.0 liters. Calculate the volume of the nitrogen gas at STP. Your response must include both a correct numerical setup and the calculated volume
What is.. 47.2 L
V2 = (273 K)(1.30 atm)(40.0 L)/(301 K)(1.00 atm)
A 3.2-gram sample of air contains 0.000 74 gram of hydrogen cyanide. Determine the concentration, in parts per million, of the hydrogen cyanide in this sample.
What is... 230 ppm or 2.31 × 102 ppm
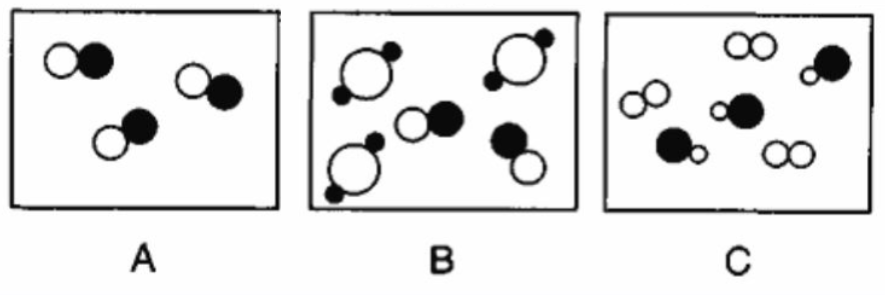
Explain, in terms of the composition, why sample A represents a pure substance.
What is.. Sample A has only one type of molecule or All particles are the same or not a mixture
Is a molecule of Bromine polar or nonpolar? Explain in terms of charge distribution.
What is... nonpolar. The molecule has an even distribution of charge.

Compare the number of molecules found in Balloon #1 to the number of molecules found in Balloon #4.
What is.. the number of molecules is the same.
Based on Table G, state the general relationship between solubility and temperature of an aqueous NH3 solution at standard pressure.
Identify the noble gas that has atoms with the same electron configuration as the metal ions in cesium chloride, when both the atoms and the ions are in the ground state.
What is.. Xenon
Write the nuclear equation for the decay of radium-226
What is alpha decay (natural transmutation)
Explain why the structure shown below for calcium chloride is incorrect.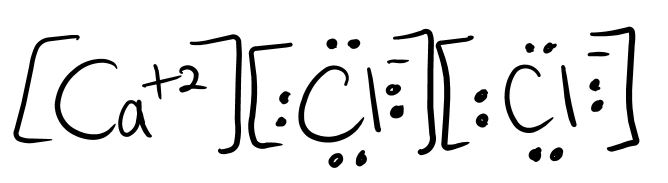
According to the Kinetic Molecular Theory of Gases, why would a balloon filled with air expand if it were heated?
What is... the motion of the gases would increase and exert a greater pressure on the inside of the balloon, causing it to expand.
Explain, in terms of the molecular polarity, why ethylene glycol dissolves in water to form a solution.
What is.. ethylene glycol and water have the same polarity.
Pb(NO3)2(aq) + KI(aq) --> PbI2(s) + KNO3(aq)
Write a chemical name for the solid product.
What is.. Lead (II) Iodide
A 3.2-gram sample of air contains 0.000 74 gram of hydrogen cyanide. Determine the concentration, in parts per million, of the hydrogen cyanide in this sample.
What is... 230 ppm or 2.31 × 102 ppm
Based on Table H, state the vapor pressure of ethanol at 75°C.
What is.. 84 kPa - 87 kPa
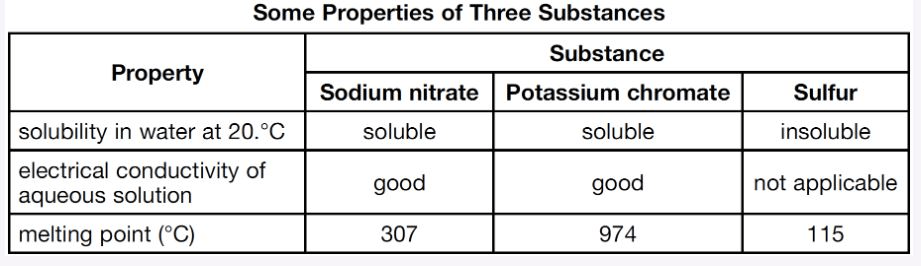
Explain, in terms of ions, why an aqueous solution of potassium chromate conducts an electric current.
What is.. The Kr2CrO4 dissociated into mobile ions
The diagram below represents three elements in Group 13 and three elements in Period 3 and their relative positions on the Periodic Table.
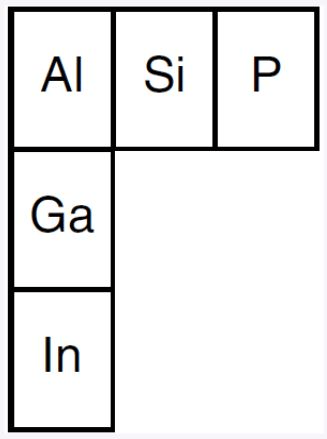
Identify one element from the diagram that will react with phosphorus in the same manner as aluminum.
What is.. – Ga, – indium, – element 31, – element 49
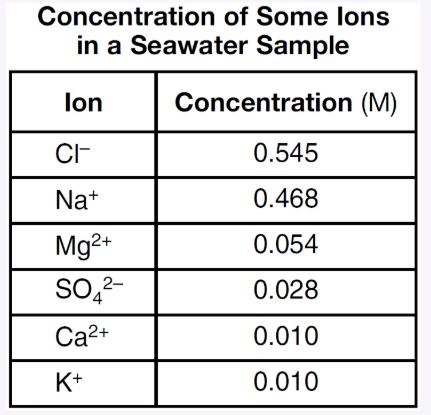
Write a chemical formula of one compound formed by the combination of K+ ions with one of these ions as water completely evaporates from the seawater sample.
What is... KCl or K2SO4
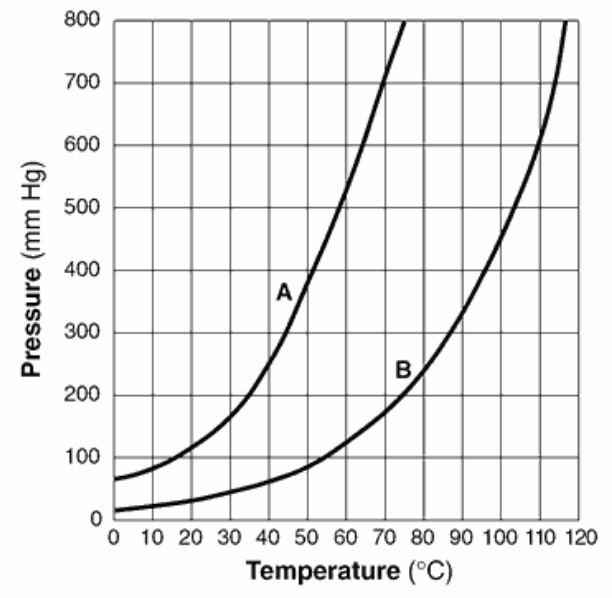
Which liquid will evaporate more rapidly? Explain your answer in terms of intermolecular forces.
What is.. Liquid A. It has weaker intermolecular forces between its particles.
Show a correct numerical setup for determining how many liters of a 1.2 M solution can be prepared with 0.50 mole of C6H12O6.
Compare the radius of a chlorine atom to the radius of a Cl– ion.
What is.. The chlorine atom is smaller than a chloride ion.