Which bond is made of a metal and non-metal?
ionic bond
What is the prefix for 4?
tetra-
What does the double line between C an O represent?
2 double bonds, 4 electrons.
How many significant figures are there in 17.600?
5 significant figures
C₃H₈(g) + 5O₂(g) → 3CO₂(g) + 4H₂O(g)
How many moles of H₂O would be produced if there were 20 moles of O₂?
16 mols of H2O
Which type of bond is C12H22O11?
Name Na₂CO₃.
sodium carbonate
How many electrons are there in the following LDS of PCl3?
26 electrons
10 lone pairs, 3 bonds
What are the products of a combustion reaction?
water and carbon dioxide
C₂H₅OH(l) + 3O₂(g) → 2CO₂(g) + 3H₂O(g)
How many moles of H₂O would be produced if there were 9 moles of O₂
9 moles of H2O
Which bond type has an electronegativity between 0.0 and 0.4?
non-polar covalent
Name NH₄OH.
Ammonium hydroxide.
Draw a LDS for Ba.

How many molecules are there in 1 mole of any substance?
6.022 * 1023 particles / mol
C₃H₈(g) + 5O₂(g) → 3CO₂(g) + 4H₂O(g)
How many liters of H₂O are produced when there are 12.5 moles of O₂
224 L of H2O
Why do metallic bonds conduct electricity?
electrons pass through metallic bonds as a "sea of electrons"
What is the formula for Cl₂O₇?
dichlorine heptoxide
Draw the LDS for a magnesium ion.
How many grams are in 1 mole of Zinc?
65.409 g Zn
C₃H₈(g) + 5O₂(g) → 3CO₂(g) + 4H₂O(g)
How many grams of CO2 are there in this reaction?
132.03 g CO2
How do the electrons in an ionic bond behave compared to a covalent bond?
ionic bonds transfer electrons while covalent bonds share them.
What is the formula for nitrogen (IV) oxide?
NO2
Draw the LDS for MgO.
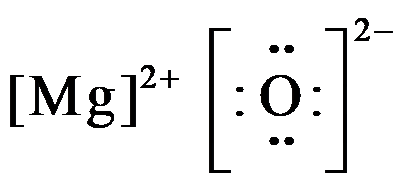
6 mols of He gas
C₃H₈(g) + 5O₂(g) → 3CO₂(g) + 4H₂O(g)
How many particles of CO₂ are produced when there are 10 moles of O₂?
3.613*10^23 particles
