A 64-year-old man is complaining of progressive dyspnea without a clear explanation. At the peak of a symptom limited exercise test, his Vo2 is 1350 mL/min (62% predicted), his minute ventilation is 55 L/min, the arterial pH is 7.27, the Pco2 is 47 mm Hg, and the Po2 is 80 mm Hg on room air. His pretest spirometry shows an FEV1 of 3.0 L (81% predicted) and a measured maximum voluntary ventilation (MVV) is 49 L/min. His heart rate at peak exercise is 71% predicted. His BP at rest is 124/70 mm Hg and goes to 178/78 mm Hg at end exercise. Which of the following patterns does this most likely represent?
A. Poor Effort
B. Ventilatory Limit from NM impairment
C. Ventilatory Limit from ILD
D. Gas Exchange Limit
Correct Answer: B
TLDR version:
This case highlights the evaluation of cardiopulmonary exercise testing (CPET) parameters to identify ventilatory, gas exchange, or cardiovascular constraints to exercise tolerance.
1. The patient shows a reduced exercise capacity, with a maximum VO2 at 62% predicted. A primary ventilatory limitation is evident, with the exercise ventilation exceeding his measured MVV
2. The MVV is commonly used as a reference for maximal exercise ventilation with normal subjects generally requiring less than 70-80% of this capability to reach maximal exercise capacity.
3. A low MVV can be caused by low lung volumes (ILD), poor effort or NM limitation. Since the patient is not achieving his predicted MVV (35-40 x FEV1), the problem is likely either poor effort or NM limitation in this case (not ILD).
4. The fact that this limitation appears to limit his ability to lower his PCO2 to buffer the developing metabolic acidosis from exercise induced lactate production suggests an impaired neuromuscular reserve (choice B) rather than poor effort (Choice A).
5. Normal oxygenation and cardiovascular parameters rule out gas exchange and cardiac causes.
Long Version:
These exercise data are a focused report addressing key elements. This patient has a significant limitation in exercise capacity as evidenced by a maximum Vo2 of only 62% predicted. A common approach to determining the cause of the exercise limitation is to evaluate ventilatory, gas exchange, and cardiovascular factors. The most obvious limiting factor in this patient involves the ventilatory system where we see that his exercise ventilation exceeds his measured MVV. Moreover, this limitation appears to limit his ability to lower his Pco2 to buffer the developing metabolic acidosis from exercise induced lactate production.
The MVV is commonly used as a reference for maximal exercise ventilation with normal subjects generally requiring less than 70-80% of this capability to reach maximal exercise capacity. There are several causes of a reduced MVV: reduced lung volumes from lung disease, neuromuscular impairment, and poor effort. To separate these mechanisms one can compare the measured MVV with a predicted MVV based on the FEV1. Normally the MVV should be 35-40 times the FEV1 with values lower than that suggesting either impaired neuromuscular function or poor effort. This patient has a predicted MVV of at least 35 x 3.0 (the measured FEV1) of 105 L/min, well above the measured 49. While this could reflect poor effort during the MVV maneuver, the lack of an appropriate ventilatory response as evidenced by rising Pco2 in the face of developing acidosis suggests that a neuromuscular problem exists (choice B is correct, and choices A and C are incorrect).
The normal oxygenation response to increasing exercise is to maintain ventilation-perfusion (V/Q) relationships (and thus oxygenation) up to very high levels of exercise (choice D is incorrect). Exercise desaturation (gas exchange limitation) usually reflects impaired V/Q relationships from lung disease. Interestingly, at extremely high levels of exercise in trained athletes, blood transit time through pulmonary capillaries become so short that a diffusion time limitation can produce hypoxemia.
The final mechanism to consider in patients with limited exercise tolerance is cardiovascular. Extensive cardiovascular measurements were not done in this patient but a heart rate % predicted commensurate with the Vo2% predicted and the stability of the diastolic blood pressure argue against significant cardiac abnormalities.
Which of the following pulmonary function changes are expected in a healthy 35-year-old woman in her 30th week of pregnancy (Figure 1)?
Correct Answer: B
The expected direction of pulmonary function testing changes in pregnancy is best exemplified by choice B (choice B is correct). Because of the enlarging uterus and diaphragmatic elevation as pregnancy progresses, there is a progressive decrease in the expiratory reserve volume and residual volume (RV) and, therefore, the functional residual capacity (FRC). The total lung capacity (TLC) decreases slightly, inspiratory capacity increases (due to hormonal-related relaxation of the respiratory muscles), and vital capacity does not change significantly during pregnancy. The decrease in RV with a relatively maintained TLC results in a low ratio of RV/TLC. The reduction in FRC, closure of the small airways during normal tidal breathing due to the aforementioned reduction in FRC, and an increase in oxygen consumption can result in changes in gas exchange and ventilation/perfusion matching, and the net result of these changes predisposes to rapid desaturation during endotracheal intubation. Tidal volume increases significantly during pregnancy, most likely due to a direct progesterone-mediated increase in central respiratory drive and enhancement of the hypercapnic ventilatory drive, as well as an increase in metabolic rate and CO2 production. Estrogen likely also plays a role. There is little or no change in respiratory rate during pregnancy. There are no significant changes in spirometry, including FEV1, FVC, and FEV1/FVC ratio, or airway resistance, during pregnancy. Although lung compliance does not change significantly, there is a reduction in total respiratory compliance owing to a reduction in chest wall compliance because the diaphragm is elevated due to uterine enlargement by the third trimester.
Choice A might be expected in interstitial lung disease, choice C is typical of obstructive lung disease, and choice D could be seen in some neuromuscular diseases where the RV is maintained to increased, resulting in an elevated RV/TLC ratio (choices A, C, and D are incorrect).
You are interpreting cardiopulmonary exercise test results from a 72-year-old man who underwent testing in your laboratory. The requisition notes “shortness of breath” as the indication for testing and mentions a history of well-controlled hypertension and hypercholesterolemia.
The patient relates a 2-year history of progressive shortness of breath to the point that he now needs to stop walking after 2 blocks. He has a dry cough, but no sputum production or hemoptysis, and reports no chest pain, palpitations, orthopnea, or ankle edema. He has a 10-pack-year smoking history but quit when he was 30 years old. The patient’s medication includes lisinopril and atorvastatin. His BMI is 29.4 kg/m2, and vital signs are normal. An ECG reveals normal sinus rhythm. Pulmonary function test results are shown (Figure 1).
For further objective evaluation, the patient undergoes cardiopulmonary exercise testing on an upright bicycle ergometer while breathing room air using an incremental ramp protocol of 15 W/min. Repeated arterial blood sampling was performed during the test. The cardiopulmonary exercise test results are shown (Figure 2, Figure 3, and Figure 4).
The 12-lead continuous ECG monitoring revealed no arrhythmias, significant ST segment, or T-wave changes. The patient reported discontinuing exercise because of a combination of shortness of breath and leg fatigue, and he had no chest pain, palpitations, or presyncope.



The results from these investigations are most consistent with the patient discontinuing exercise from which of the following?
A, The test cannot be appropriately interpreted because of technical reasons.
B. Deconditioning
C. Heart Failure
D. Interstitial Lung Disease
Correct Answer: D
TLDR Version:
1. This CPET demonstrates significant exercise limitation (low VO2 max, low AT, low workload) with both ventilatory and gas exchange limitations as might be expected in a patient with ILD.
2. Ventilatory limitations are demonstrated by i) markedly elevated ventilatory equivalents for both O2 and CO2, ii) blunted VT response during exercise iii) flow-volume curves demonstrating near absent inspiratory reserve volume, with minimal reserve to increase tidal volume further
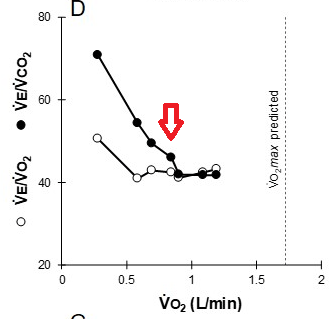
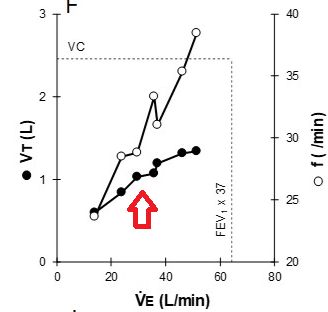
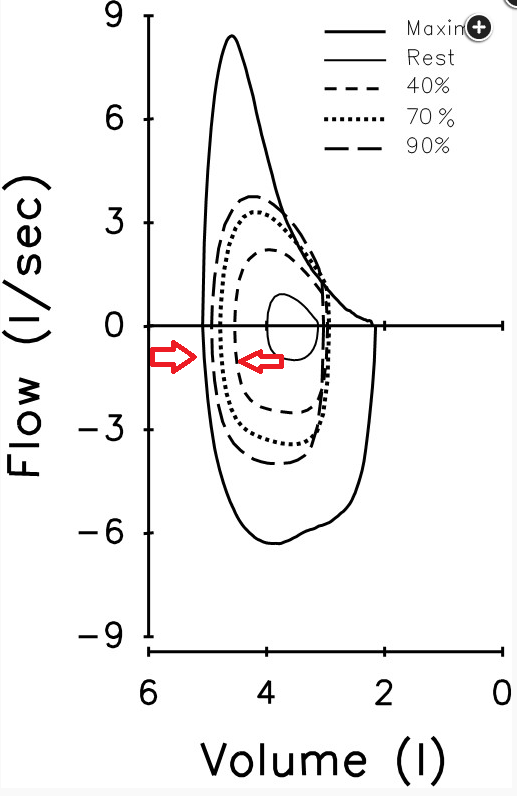
3. Gas exchange limitation is evidenced by i) desaturation to 79% at the end of exercise, and ii) a widened A-a gradient during exercise
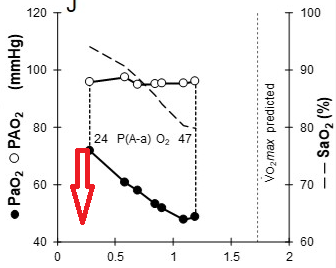
Long Version:
This patient demonstrates significant exercise limitation with a markedly reduced work capacity (end-exercise workload 66% predicted), decreased aerobic capacity (peak V̇O2 69% predicted), and a low anaerobic threshold (36% predicted maximal peak V̇O2) (Figure 2). While estimated maximal ventilatory capacity is not achieved (V̇E /MVV 80% predicted) (Figure 3, panel E, and Figure 4), V̇E is increased during submaximal exercise (Figure 3, panel E), and there are a blunted VT response during exercise (Figure 3, panel F), markedly elevated ventilatory equivalents for both O2 and CO2 (Figure 3, panel D), abnormal gas exchange with desaturation to 79% at the end of exercise, and a widened A-a gradient during exercise (Figure 3, panel J). Inspection of the flow-volume curves (Figure 4) demonstrates near absent inspiratory reserve volume, with minimal reserve to increase tidal volume further. These findings, together with the resting pulmonary function test results, are highly suggestive of interstitial lung disease (ILD) (choice D is correct).
ILD is physiologically characterized by increased elastic loading and decreased static lung compliance, leading to altered ventilatory mechanics manifested in part by a rapid, shallow breathing pattern. In addition, significant V/Q mismatch, diffusion limitation, and reduced mixed venous O2 (SvO2) contribute to impaired gas exchange. In this patient, exercise was limited by markedly abnormal gas exchange before a mechanical ventilatory limitation was achieved. Nonetheless, there is clear evidence of ventilatory inefficiency, an abnormal breathing pattern, and reduced breathing reserve at the end of exercise. Other known contributors to exercise limitation in this population include abnormal pulmonary vasculature and cardiovascular function, as well as compromised peripheral muscle function. These are difficult to assess independently in this instance. However, if exercise were repeated with the patient breathing a hyperoxic inspirate (thus reducing the physiologic manifestations of hypoxemia and decreased O2 delivery), the contribution of these other factors could potentially be better understood.
The reported results from testing seem appropriate in this instance and can therefore be interpreted as outlined—there are no apparent significant technical issues that would preclude interpretation (choice A is incorrect). Although deconditioning is a common finding in patients with exertional dyspnea, they would not display such markedly abnormal ventilatory mechanics and abnormal gas exchange (choice B is incorrect). There is no history of cardiac disease, cardiac examination is normal, and the resting ECG is normal. The lack of a tachycardic heart rate response, the normal oxygen pulse during exercise (although this can be decreased when O2 content is reduced in the setting of significant O2 desaturation or R → L shunt), the lack of other abnormalities consistent with impaired cardiac function, and the presence of characteristic findings of ILD exclude cardiac dysfunction as the etiology most responsible for exercise limitation in this patient (choice C is incorrect).
A normal subject can reduce his/her carbon monoxide diffusing capacity (Dlco) by which of the following?
A. Exercising
B. Going Supine
C. Performing a Valsalva maneuver
D. Performing a Mueller maneuver
Correct Answer: C
The two major determinants of carbon monoxide uptake by the lungs (Dlco) are alveolar-capillary membrane properties (diffusion properties and surface area – Dm) and pulmonary capillary blood volume coupled with the reaction rate of CO binding to hemoglobin (thetaVc). These determinants are roughly equal in importance and are mathematically expressed as 1/Dlco = 1/(thetaVc) + 1/Dm.
During Dlco testing, an expiratory effort against a closed glottis (Valsalva maneuver) increases intrathoracic pressure creating a decrease in pulmonary capillary blood volume (Vc). Dlco has been shown to drop up to 30% under these circumstances (choice C is correct). This is important to remember during Dlco testing and patients should be encouraged to relax during the breath-hold rather than push against the closed test valving.
In a normal seated subject, gravity produces a relative hypo-perfusion of apical lung regions – so called “West zone 1” regions. This is increased during the Valsalva maneuver as described above. In contrast, maneuvers that increase Vc and reduce zone 1 conditions (eg assuming the supine position, increasing pulmonary capillary blood flow with exercise, reducing intrathoracic pressure with an inspiratory effort against a closed glottis (Mueller maneuver) will increase Dlco (choices A, B, and D all incorrect).
You consult with a 40-year-old patient with a history of exercise-induced bronchospasm who presents with persistent dyspnea during exercise 6 months after COVID-19 pneumonia. Since recovery from her infection, she has had shortness of breath with activity and describes chest tightness and difficulty experiencing a full deep breath. Despite this, she is able to walk regularly.
She has a medical history notable for exercise-induced bronchospasm and bronchitis as a child. She works as a nurse. She does not smoke cigarettes. There is a pet cat at home. Medications include a combination β-agonist and corticosteroid inhaler, montelukast and tiotropium.
On examination, her BP is 116/75 mm Hg, heart rate is 87/min, Spo2 is 99%, and BMI is 21.5 kg/m2. She has no thyromegaly or lymphadenopathy. There was normal percussion of the thorax and movement of the diaphragm, and she has normal breath sounds without wheezes. Her heart sounds were normal, with no gallop or murmur. Her abdomen is soft and nontender, with no organomegaly. She has no ankle edema and no cyanosis or clubbing. She has normal strength in all extremities. A chest radiograph is shown in Figure 1.
The patient has undergone a cardiopulmonary exercise test with insertion of a pulmonary artery catheter. Before and after exercise spirometric results were as follows:
Before: FVC, 3.35 (120%); FEV1, 2.94 (126%); FEV1/FVC, 88 (106%)
After: FVC, 3.41 (122%); FEV1, 3.00 (129%); FEV1/FVC, 88 (106%)
She had a normal increase in tidal volume during exercise and no change in end-expiratory lung volume. The maximum predicted heart rate was 95% of predicted. There was no oxygen desaturation during exercise and no evidence of increased dead space.
Her anaerobic threshold was low. The oxygen pulse increased linearly during exercise. The peak oxygen pulse was 58% of predicted. Maximal oxygen consumption was reduced, as was anaerobic threshold. Mixed venous Spo2 was 75%. There was no change in end-expiratory lung volume during exercise. At peak exercise, ventilation was 40 L/min.
What is the most likely cause of the patient's exercise limitation?
A. Dynamic Hyperinflation due to exercise induced bronchospasm
B. Abnormal extraction and use of Oxygen
C. Maximal ventilation achieved
D. Pulmonary vascular disease
Correct Answer: B
TLDR:
This patient shows reduced maximal oxygen consumption and low anaerobic threshold, suggesting an oxygen extraction and use defect, likely related to microcirculation or mitochondrial issues. This pattern, observed in some post-COVID-19 patients with persistent exertional dyspnea, is supported by a high mixed venous O2 level, indicating that cardiac output is not limited. The lack of changes in oxygen pulse and normal heart size further rule out stroke volume or cardiac abnormalities.
Despite a history of exercise-induced asthma, the patient shows no exercise-induced changes in spirometry, ruling out bronchoconstriction or flow limitation. Pulmonary vascular disease is also unlikely, as this condition would cause oxygen desaturation, increased dead space, and low cardiac output.
Long Version:
Maximal oxygen consumption is reduced, and anaerobic threshold is low. Given the high mixed venous O2, this is unlikely due to a reduction in cardiac output. In the absence of a history of cardiac disease, a normal-size heart on a chest radiograph in a young person, and no blunting of the oxygen pulse curve during exercise, there is no evidence for an underlying abnormality with stroke volume and oxygen delivery. The low peak oxygen consumption, consequently, in conjunction with the high mixed venous Spo2, is consistent with an oxygen extraction and use defect, which has been reported in some patients with post-COVID-19 exertional dyspnea. This has been postulated to be due to a problem in the microcirculation or possibly in mitochondrial function (choice B is correct).
The oxygen pulse is a surrogate marker for stroke volume in many cases, although it may be low due to a problem with oxygen extraction as well. Blunting or leveling off of the oxygen pulse, which is not present in this case, is indicative of acute ischemia leading to reduced stroke volume and greater reliance on heart rate to increase cardiac output and oxygen delivery.
Although the patient has a history of exercise-induced asthma and complains of chest tightness, a common sensation characterizing dyspnea due to bronchoconstriction, the patient is being treated with multiple bronchodilators and asthma controller medications, and there is no change in spirometric results during exercise, which might be expected with acute bronchoconstriction. There is no change in end-expiratory lung volume, which argues against flow limitation and dynamic hyperinflation (choice A is incorrect).
The patient did not achieve maximal ventilation (typically estimated as 35 × FEV1 at the time of peak exercise); thus, there was no ventilatory limit to exercise (choice C is incorrect).
Pulmonary vascular disease sufficient to account for the exercise limitation in this case would likely be associated with oxygen desaturation and a low cardiac output leading to reduced mixed venous Spo2. Increased dead space would also be noted (choice D is incorrect).
Persistent dyspnea in patients after COVID-19 may be due to a variety of problems, including interstitial fibrosis, thromboembolic disease, cardiovascular deconditioning, and reduced cardiac function from myocarditis, as well as the issue of impairment of O2 extraction. Results from some studies have also suggested weakness of diaphragmatic contraction. Additional hypotheses include reduced perception of lung expansion, possibly because of neuropathy. Further research may help delineate these factors in the years to come. Cardiopulmonary exercise testing may help distinguish these causes.
Which of the following decreases during a normal pregnancy?
A. Minute Ventilation
B. Vital Capacity
C. Serum Bicarbonate
D. Arterial pH
Correct Answer: C
Gas exchange in pregnancy is characterized by a mild compensated respiratory alkalosis secondary to an increase in minute ventilation. Thus, normal Paco2 values are lower, 28 to 32 mm Hg, and serum bicarbonate is compensatorily decreased to 18 to 21 mEq/L (18 to 21 mmol/L) (choice C is correct). Normal pH is mildly alkalemic at 7.40 to 7.45 (choice D is incorrect). Pao2 is slightly elevated, 100 to 105 mm Hg, with a slight decrease at term to 100 mm Hg. There is normally a 5 to 10 mm Hg elevation in alveolar-arterial gradient above baseline, especially in the supine position. Oxygen consumption increases by 20% to 30% during pregnancy, with a concomitant increase in carbon dioxide production by 30% to 35%. These changes are due to increased maternal and fetal metabolic requirements and increases in work of breathing and cardiac output.
The major change in pulmonary function testing in pregnancy is a progressive decrease in the expiratory reserve volume by 8% to 40% and a decrease in residual volume by 7% to 22%, both changes due to the enlarging uterus and diaphragmatic elevation. Because of these changes, functional residual capacity is, in turn, decreased by 10% to 25% by the third trimester of pregnancy. Total lung capacity may decrease slightly, inspiratory capacity increases, and vital capacity does not change significantly during pregnancy (choice B is incorrect). The decrease in residual volume with a relatively maintained total lung capacity results in a low ratio of residual volume to total lung capacity. Closure of the small airways during normal tidal breathing due to the reduction in functional residual capacity can result in changes in ventilation/perfusion matching and gas exchange. Tidal volume increases significantly during pregnancy by 150 mL to a final value of 450 to 600 mL, or a 30% to 50% increase in the average patient. This is most likely due to a direct progesterone-mediated increase in central respiratory drive and enhancement of the hypercapnic ventilatory drive. There is little or no change in respiratory rate during pregnancy, and tachypnea is an unusual finding. Because of the increase in tidal volume, there is a significant increase in resting minute ventilation from 6 L in the nonpregnant state to 9 L at full term (or 20% to 50% above baseline) (choice A is incorrect). There are no significant changes in peak flow rates, FEV1, airway resistance, or maximum voluntary ventilation during pregnancy. Although lung compliance does not change significantly during pregnancy, there is a reduction in total respiratory compliance due to a reduction in chest wall compliance because of an elevated diaphragm caused by uterine enlargement by the third trimester. Diffusing capacity of the lung for carbon monoxide may increase slightly in the first trimester followed by a slight decrease later in pregnancy, likely due to alterations in pulmonary vascular volume.
The cardiovascular system probably undergoes the most significant changes during pregnancy, including an increase in cardiac output, beginning in the first trimester and peaking at about the 25th to 32nd week at 30% to 50% above normal. This is due to both a change in heart rate as well as stroke volume, and a decrease in systemic vascular resistance, partially due to shunting of blood to the low-resistance placental bed and perhaps due to increased levels of vasodilator mediators. Pulmonary vascular resistance also decreases. Both systolic and particularly diastolic pressures are reduced. Postural hypotension may be apparent, particularly in the third trimester when the uterus can compress the inferior vena cava, impeding venous return to the heart.
A 51-year-old woman is referred for cardiopulmonary exercise testing for evaluation of dyspnea not yet diagnosed. She jogs approximately 8 km/day and recreationally participates in half-marathons, and she has noticed increased shortness of breath while running, which is affecting her performance. She has tried albuterol but has not noticed much improvement. There is no associated cough or wheezing, and she has no history of asthma, allergic rhinitis, or atopy. She reports no chest pain, palpitations, syncope or near-syncope, or light-headedness. She mentions she works in a stressful environment as an ED nurse. Her history is otherwise negative, she is a lifelong nonsmoker, and she is on no medications. Her BMI is 22 kg/m2, and physical examination is normal. Pulmonary function test results are shown in Figure 1.
A methacholine challenge test demonstrated a PC20 (percent concentration associated with a 20% fall in the FEV1) of >32 mg/mL. Her symptoms were not reproduced during the methacholine challenge test. The patient subsequently underwent cardiopulmonary exercise testing on an upright bicycle ergometer while breathing room air and using an incremental ramp protocol of 20 W/min. The cardiopulmonary exercise testing results are shown (Figure 2, Figure 3, and Figure 4).
Twelve-lead continuous ECG monitoring revealed no arrhythmias or ST segment or T wave changes. The patient reported discontinuing exercise because of a combination of shortness of breath and leg fatigue, and she had no chest pain, palpitations, or presyncope. Results from spirometry performed immediately after exercise are shown in Figure 5.




The results from these investigations are most consistent with which of the following?
A. Vocal Cord Dysfunction
B. Mitochondrial myopathy
C. Exercise induced bronchoconstriction
D. Primary hyperventilation syndrome
Correct Answer: C
TLDR:
The patient, with exertional shortness of breath but normal PFTS and CPET performance values, was found to have a 14% drop in FEV1 after exercise, indicating exercise-induced bronchoconstriction (EIB). EIB can occur even without typical asthma symptoms and may not be excluded by a negative methacholine challenge test. Diagnosis of EIB relies on post-exercise spirometry, which should be done at set intervals after exercise to measure FEV1. A 10% or greater decrease in FEV1 confirms EIB, with severity graded by the degree of FEV1 reduction. In this case, no signs of central airway obstruction, mitochondrial myopathy, or hyperventilation syndrome were observed, ruling out alternative diagnoses. A proper testing protocol involves short, intense exercise without prolonged warm-up to prevent tolerance to EIB.
Long Version:
This patient presents with shortness of breath with exertion in the setting of normal pulmonary function and a normal methacholine challenge test. Cardiopulmonary exercise testing revealed normal performance and values, except for a 14% decrease in the FEV1 from the pre-exercise value (3.51 to 3.03 L) (Figure 5) immediately following exercise consistent with a diagnosis of exercise-induced bronchoconstriction (EIB) (choice C is correct). Exercise-associated airway narrowing occurs in most patients with asthma. Although patients often deny or do not recognize other symptoms of asthma, these symptoms can often be detected with a careful clinical history. A diagnosis of EIB is not excluded by a negative methacholine challenge test, although it is commonly positive in this clinical setting. In this instance, the patient underwent cardiopulmonary exercise testing to understand objectively the patient’s symptoms of exertional dyspnea and impaired exercise performance, which was confirmed to be normal (the patient demonstrated normal work and aerobic capacity) (Figure 2). However, spirometry following exercise (Figure 5) revealed a significant decrement in the FEV1, which subsequently responded to the administration of a bronchodilator.
A suggested postexercise spirometry testing schedule is 1, 3, 5, 10, 15, 20, and 30 min after cessation of exercise. It is recommended to assess FEV1 because this measurement has better repeatability and is more discriminating than peak expiratory flow rate. A significant drop in FEV1 following exercise confirms the diagnosis of EIB, although if these results were not demonstrated with this incremental protocol and clinical suspicion for EIB remained, a more specific EIB exercise protocol would have been indicated. This typically consists of high-intensity exercise on a treadmill or bicycle ergometer for 6 to 8 min intended to achieve rapidly the highest possible level of ventilation for 4 to 6 min. The ventilation required for a valid test is at least 17.5 times FEV1 and preferably >21 times FEV1.There should not be a significant warm-up period, which may lead to tolerance or refractoriness to EIB, which can also occur if exercise duration exceeds 12 min (as potentially with running a half-marathon). A fall in the FEV1 of 10% or more is interpreted as abnormal. Severity may subsequently be graded as mild (>10% but <25%), moderate (>25% but <50%), or severe if the fall in FEV1 from the pre-exercise is >50%. These bronchoconstriction responses may also occasionally be demonstrated with eucapnic voluntary hyperventilation; cold air challenge; hyperosmolar aerosols, including 4.5% saline; and dry powder mannitol.
Not mentioned in the information provided is that the patient’s symptoms were also reproduced immediately following exercise; this is a common finding and helps to strengthen the diagnosis further. However, the diagnosis of EIB is established by changes in lung function provoked by exercise, not based on symptoms. A significant fall in the FEV1 may also be seen in patients with upper airway obstruction or vocal cord dysfunction, and these cases can be readily distinguished from EIB by examination of the exercise tidal flow-volume curves. In this patient, the tidal exercise flow-volume curves, both at rest and with exercise, do not reveal any changes consistent with central airway obstruction (choice A is incorrect). Patients with mitochondrial myopathies characteristically demonstrate significant aerobic and work impairment (choice B is incorrect). While the patient works in a stressful occupation, there are no findings consistent with primary hyperventilation syndrome, such as an erratic breathing pattern or hyperventilation that is excessive for the simultaneous metabolic load (choice D is incorrect).
A 35-year-old man celebrating his 10-year wedding anniversary travels to Bali with his spouse. There they embark on a 3-day recreational scuba diving trip that includes a 35-ft (10.5 m) dive to view a shipwreck on the final day. On returning to the water surface, he notes some nonspecific left hip and shoulder pain that he attributes to being “out of shape.” Onboard the boat to return to shore, he begins to experience a bi-temporal headache and a sense of mild pruritus. He is otherwise well, without significant medical history. On arrival to shore, he is seen by the diving company physician. He is taking no prescription medications but took two ibuprofen tablets on the boat ride to shore, and his headache resolved. Physical examination reveals normal range of motion of the extremities, with tenderness of the left hip and shoulder, normal thoracic movement, and clear breath sounds on auscultation. Skin examination is notable for a tattoo of an eagle on his left deltoid region and mild sunburn without signs of rashes. His neurological exam is normal.
Which of the following interventions would be the next most appropriate step in his management?
A. Fly to a nearby island where a specialty hospital is accessible
B. Limit fluids to no more than 50 mL/h over the next 8 h
C. Initiate acetazolamide 250 mg orally every 8 h
D. Start high-flow supplemental oxygen
Correct Answer: D
The presence of spontaneous joint pain, particularly in the larger joints such as the hips, shoulders, knees, and elbows, combined with headache, extremity tingling, and pruritus after diving at relative depth greater than 2 atm absolute (>33 ft [10 m]) are all signs of type I decompression sickness (DCS), also known as "the bends." The next most appropriate therapy in this case would be to administer high-flow supplemental oxygen (100% FiO2) immediately (choice D is correct).
DCS occurs when dissolved gases (mostly nitrogen) come out of solution and form bubbles inside the body on depressurization. This is based on Henry's law that describes the solubility of a gas in a liquid is proportional to the partial pressure of the gas over the liquid. As divers breathe using compressed air at depth, more nitrogen dissolves into the blood. With the greater intrathoracic pressure experienced while diving, gases such as nitrogen, helium (when used in diving gas mixtures), and oxygen become increasingly soluble in the blood. Unlike oxygen that is metabolized, nitrogen and helium build up throughout the body. When divers want to emerge from the water, ascents must be performed gradually, since bubbles may form rapidly if pressure changes occur too quickly, much like a carbonated beverage bubbling on opening its sealed cap or pull tab. These bubbles may lodge in joints, the pulmonary parenchyma, the skin, or the vascular system. Placing the patient on high-flow 100% supplemental oxygen creates an oxygen-rich, nitrogen-poor environment, resulting in a gradient of nitrogen between the blood and the bubble, causing nitrogen to move from the bubble into the bloodstream, which, in effect, shrinks or resolves the bubbles. This process of driving nitrogen into solution is accelerated with hyperbaric oxygen therapy (HBOT). Although DCS occurs most commonly from underwater diving decompression as an individual ascends, occupations in which workers perform physical maneuvers in a caisson or performing extravehicular activity from spacecraft are other environments in which DCS is prevalent. Proper decompression procedures during diving can help decrease the risk for developing DCS, which is a relatively rare event, estimated at three occurrences in 10,000 dives. DCS is more common in men than women, and the shoulders are the joints most commonly affected.
DCS has been classified as type I (symptoms involving only the skin, musculoskeletal system, or lymphatic system), and type II involves more severe symptoms that involve the CNS. Skin mottling, discoloration, or marbling has been referred to as "cutis marmorata" and is the most common visible skin condition encountered. This may be preceded by a sense of generalized pruritus.
Although symptomatic DCS patients should be treated with HBOT as soon as feasible, flying in an unpressurized aircraft (such as a helicopter) would not be recommended as the first step in therapy, since symptoms may be exacerbated. If an individual with more serious symptoms must be mobilized to a specialty medical center for treatment, unpressurized aircraft should remain below 1,000 ft (300 m), or a pressurized aircraft should be used for transport (choice A is incorrect). A standard chest radiograph should be obtained prior to HBOT to rule out a pneumothorax, since untreated pneumothoraces could be lethal due to the potential conversion into a tension pneumothorax during therapy, since the intrapleural air expands on decompression, and lack of accessibility to the patient for a period of time while undergoing hyperbaric therapy in a pressurized chamber makes emergency maneuvers challenging. Although various organizations have published suggested varying lengths of time to wait until flying after diving, the longer the time interval, the less risk of developing DCS. For example, after dives not requiring decompression stops during ascent, a minimum preflight surface interval of 12 h is suggested, while after multiple no-decompression dives per day or multiple days of diving, a minimum preflight surface interval of 18 h is suggested.
One of the risk factors for developing DCS is dehydration. Lack of intravascular volume may result in headaches, irritability, confusion, fatigue, muscle cramps, and reduction of thermoregulation. Dehydration results in reduced tissue perfusion; if the reduction in blood plasma becomes excessive, gaseous exchange becomes less efficient, so nitrogen off-gassing is less efficient, with risk of DCS increasing. Therefore, limiting oral hydration or prescribing diuretics for DCS treatment is contraindicated (choices B and C are incorrect).
A 54-year-old woman is referred for cardiopulmonary exercise testing (CPET) related to investigation of exertional shortness of breath and activity limitation. Her symptoms began 3 years prior when she noticed difficulty participating in adult recreational soccer, and she now finds herself short of breath after climbing two or three flights of stairs. She also notes cough for the past 5 years and occasional wheezing with vigorous exertion. She tried albuterol for her symptoms but did not notice any improvement. She reports no hemoptysis, sputum production, chest pain, orthopnea, or palpitations. The remainder of the clinical history is negative except for periodic heartburn that responds to antacids and diet modification. She is currently on no medications and is a lifelong nonsmoker. Her BMI is 24.7 kg/m2, her vital signs are normal, and there are no significant murmurs or wheezes on physical examination. An ECG and chest radiograph are normal. Pulmonary function test results are shown (Figure 1).
The patient undergoes CPET on an upright bicycle ergometer while breathing room air using an incremental ramp protocol of 15 W/min. CPET results are shown (Figure 2, Figure 3, and Figure 4).
Monitoring with 12-lead continuous ECG revealed no arrhythmias, significant ST segment, or T-wave changes. The patient reported discontinuing exercise because of shortness of breath, and she had no complaints of chest pain, palpitations, or presyncope. Spirometry performed following exercise was unchanged from pre-exercise values.



The results from these investigations are most consistent with the patient discontinuing exercise from which of the following?
A. Central Airway obstruction and ventilatory limitation
B. Deconditioning
C. Normal Exercise physiology
D. ionotropic cardiac dysfunction
Correct answer: C
This patient demonstrates normal work (end-exercise workload >90% predicted) and aerobic capacity (peak V̇O2 >90% predicted), a normal anaerobic threshold (>40% predicted maximal peak V̇O2), near maximal heart rate (>90% predicted maximal heart rate), and an appropriate increase in BP during exercise with a normal O2 pulse. In addition, there is significant ventilatory reserve at the end of exercise (V̇E /MVV 49% predicted) (Figure 2 and Figure 3, panel E), normal gas exchange with no oxygen desaturation, and no evidence of significant expiratory or inspiratory flow limitation on tidal flow-volume (f-v) curves during exercise (Figure 4). The breathing pattern during exercise is normal (Figure 3, panel F), the ventilatory equivalents for both O2 and CO2 are normal (Figure 3, panel D), and estimated dead space ventilation falls appropriately during exercise (Figure 3, panel G). This constellation of findings, with the absence of other significant abnormalities, is most consistent with normal exercise physiology and performance (choice C is correct). Even though work and aerobic capacity are normal, it is possible this patient could have exercised more as heart rate reserve was still present at the end of exercise (Figure 2).
The resting f-v curve is abnormal (Figure 4), with flattening of the expiratory curve at a flow of approximately 3.5 L/min. The maximal inspiratory curve does not demonstrate such flattening, so this is suggestive of variable intrathoracic central airway obstruction. But, importantly, in this patient, the maximal tidal f-v curve (Figure 4, dashed line) does not encroach on the maximal curve (Figure 4, solid thick line) during exercise—that is, there is expiratory flow reserve at the end of exercise (choice A is incorrect). It is possible the complex sensation of dyspnea experienced by this patient may be heightened because of the impaired maximal expiratory flow, but this finding is not physiologically limiting the patient’s exercise performance.

Figure 5 demonstrates findings on exercise tidal f-v curves characteristic of significant central airway obstruction. There is expiratory and inspiratory flow limitation at mid-exercise (dotted line) such that further increases in ventilation at the end of exercise (dashed line) are achieved only by a horizontal decrease in end-expiratory lung volume and an increase in end-inspiratory lung volume—that is, maximal expiratory and inspiratory flows do not change after mid-exercise.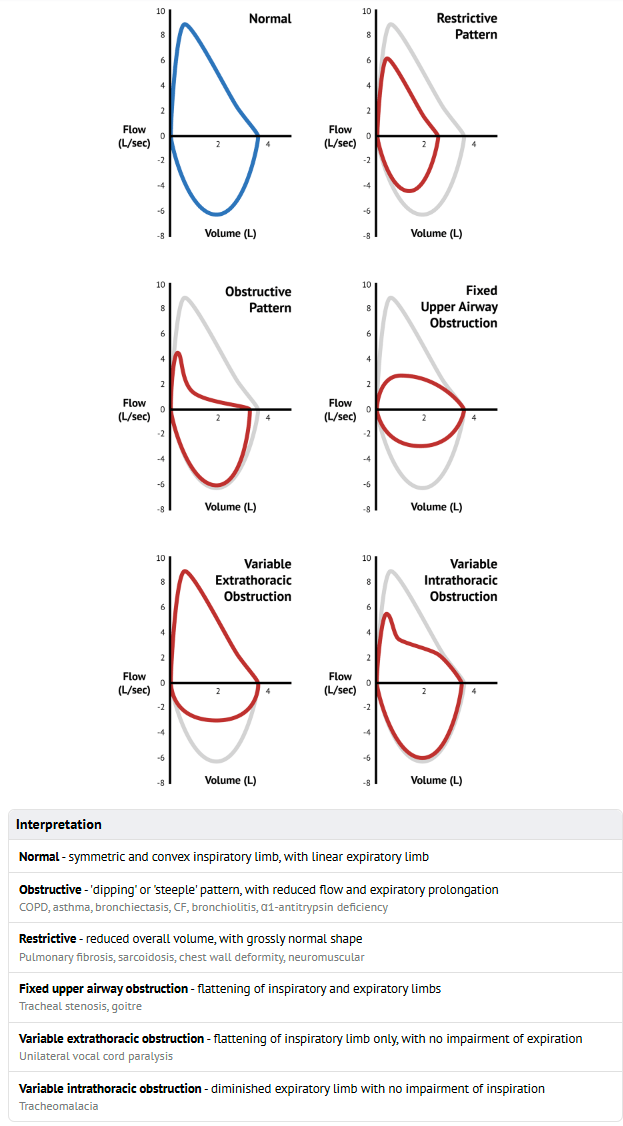
Although deconditioning is a common finding in patients with unexplained exertional dyspnea, this patient exhibited normal work and aerobic capacity (choice B is incorrect). Patients with deconditioning also more characteristically demonstrate an exaggerated heart rate during exercise unless impaired exercise performance is associated with a physiologically submaximal effort. There is no history of cardiac disease, cardiac examination is normal, and the resting ECG is normal. The lack of a tachycardic heart rate response, the normal oxygen pulse during exercise, and the lack of other abnormalities consistent with impaired cardiac function exclude inotropic cardiac dysfunction as the etiology most responsible for exercise limitation in this patient (choice D is incorrect).
This patient underwent additional investigations that led to a diagnosis of subglottic stenosis of unknown etiology, although significant gastroesophageal reflux was documented. Treatment of the gastroesophageal reflux did lead to near-complete resolution of her cough. She was been followed up for 1 year after these investigations, and her clinical status remains stable.
A 60-year-old woman with a medical history of severe OSA, hypothyroidism, and hypertension reports to you that during a recent 7-day trip to Vail, Colorado (altitude 8,120 ft [2,436 m]), she developed excessive daytime sleepiness and worsening of sleep quality. She has been using auto-CPAP very adherently with a nasal mask interface for the last 5 years, and the device is set at a minimum pressure of 5 and a maximum pressure of 12 cm H2O. Hypothyroidism and hypertension are well controlled with levothyroxine and hydrochlorothiazide, respectively. She routinely monitors the performance of her auto-CPAP device on her mobile application. While living in Chicago (altitude 597 ft [179 m]), she uses auto-CPAP very adherently for a mean usage of 7 h 15 min per night, and the device-estimated residual apnea-hypopnea index is 1.5 events per hour. During her Vail sojourn, she noticed that the device was reporting an elevated residual apnea-hypopnea index of 25 events per hour. Additional data from the download of her auto-CPAP device is presented (Figure 1).
She is planning to travel again to Vail and is hoping to avoid worsening of her sleep quality and development of daytime sleepiness so that she can better enjoy the vacation with her family. What do you recommend?
A. Adjusting auto-CPAP settings from 5 to 12 cm H2O to 7 to 15 cm H2O
B. Switching to a bilevel positive airway pressure device
C. Taking acetazolamide 250 mg twice a day
D. Switching the nasal mask to an oronasal mask
Correct Answer: C
In patients with untreated OSA, sojourn to a high altitude can significantly worsen sleep-disordered breathing. The exacerbation of sleep-disordered breathing is driven primarily by development of central sleep apnea, not worsening of OSA. Hypobaric hypoxia at altitudes >6,666.67 ft (>2,000 m) induces periodic breathing with central apnea. Breathing instability is related to hypoxic stimulation of ventilation, resulting in a reduced CO2 reserve—that is, eupneic PCO2 close to the apneic threshold promoting a central apnea at a minor rise in ventilation. Enhanced chemosensitivity causes a ventilatory overshoot, leading to a vicious cycle of periodic breathing with central apneas. In a Swiss study of patients with OSA, the mean apnea-hypopnea index (AHI) increased from 51 to 89 events per hour after 2 days at a high altitude of 8,633.33 ft (2,590 m). The obstructive AHI did not change significantly, but the central apnea index increased from two to 29 events per hour. During altitude sojourns, combined treatment with acetazolamide and auto-CPAP, compared with auto-CPAP alone, resulted in improvement in nocturnal Spo2, better control of sleep apnea, and reduced insomnia (choice C is correct). In this study, acetazolamide was initiated on arrival at the high-altitude destination and was prescribed as 250 mg every morning and 500 mg every evening, prior to meals.
In the current case, a more detailed download of the patient's auto-CPAP device revealed that the estimated AHI of 25 events per hour while at high altitude consisted of an obstructive AHI of 2 and a central apnea index of 23 events per hour. The reason this patient's auto-CPAP device did not increase the delivered pressure while at high altitude is because the device is not designed to increase CPAP pressure in response to central apneas. Therefore, preemptively increasing the range of the auto-CPAP device will not solve the patient's problem (choice A is incorrect). Switching to a bilevel positive airway pressure (PAP) device will not solve the problem because, in fact, bilevel PAP therapy will lead to larger tidal volume delivery and will further decrease the PaCO2, thereby increasing respiratory instability and worsening of central apneas. Moreover, it is not practical to switch the device to bilevel PAP because insurance providers will not cover the cost (choice B is incorrect). The download reveals that the amount of large leak is minimal. Switching from a nasal mask to an oronasal mask is unnecessary; in some patients, it can increase discomfort and even worsen OSA by pushing the chin and the tongue backward, inducing upper airway obstruction (choice D is incorrect).