For an ideal gas at constant volume, as temperature increases, pressure ________________ .
What is increases?
If ΔH of a reaction is less than zero than reaction will be exothermic, endothermic, or unknown?
What is exothermic?
The process of going from a solid to a gas is known as this.
What is sublimation?
Overall, ΔSuniverse is always ______________ .
What is increasing?
The following reaction is elementary as written:
NH2 + H --> NH3
What is the rate law?
What is rate = k[NH2][H]
This happens to the peak HEIGHT for the following distribution of gas particles when the temperature is increased.

What is decreases!
(Peak height decreases, curve moves to right)
The ΔH of vaporization is the following value.
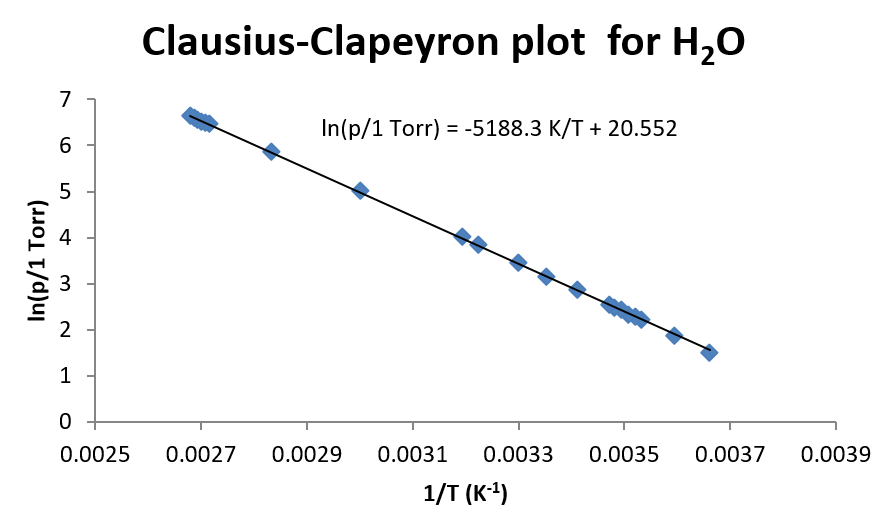
What is 43135 J/mol (or 43.1 kJ.mol)
The normal boiling point of a substance is its boiling point at this pressure.
What is 1 atm?
If in a certain reaction, ΔH is negative and ΔS is positive, will this reaction be spontaneous, non-spontaneous, or unknown?
What is spontaneous?
The Arrhenius equation, can be used to calculate this energetic quantity.
What is the activation energy of a reaction?
The partial pressure of helium if it exists in a mixture of 32 g of oxygen and 36 g of helium has a total pressure of 2.0 atm.
What is 1.8 atm.
1) Calculate mols of Helium and Oxygen (9 and 1 respectively)
2) Calculate mol fraction of Helium (9/10 = 0.9)
3) Multiply by total pressure (0.9*2)
In an isochoric process the increase in internal energy (ΔE) is equal to this.
What is the heat absorbed (or positive q)?
Assign the appropriate labels for the below phase diagram
A -> gas
B -> solid
C -> Liquid
D -> Triple point
Arrange the following in order of decreasing molar entropy at 298 K.
HCl CH4 Ar
What is CH4 > HCl > Ar?
(more molecular complexity = higher entropy)
The rate of a chemical reaction is as follows:
rate = k([A][B]2)/([C]1/2[D]1/2)
What happens to the reaction rate if the concentrations of A, C, and D are all doubled?
What is stay the same?
The units of the gas constant R, if its value is 8.314.
What is J/mol.K
(also accepted:
m3⋅Pa/mol.K and its variations)
In a bomb calorimeter, this reactant is provided in excess.
What is Oxygen gas?
What is ΔH of the below transformation?
C (s, graphite) ---> C (s, diamond) ΔH° = ??? kJ
----------------------------------------------------------
C (s, graphite) + O2(g) ---> CO2(g)
ΔH° = −393.5 kJ
C (s, diamond) + O2(g) ---> CO2(g)
ΔH° = −395.4 kJ
What is +1.9 kJ?
10 particles exist in container 1. Over time, these particles distribute into container 2 where 5 particles exist in each container. What is the total number of microstates (W) present?
(N!/(n1!n2!)) = (10!/(5!5!))
The following plot shows a reaction that is _____________ order in A.
What is second order?
The rate of effusion of an unknown diatomic gas is 5 mL/min. Under identical conditions the rate of effusion of hydrogen gas is 20 mL/min.
What is the unknown gas?
What is O2?
(Graham's Law of Effusion: 20/5 = sqrt(MM unknown/ MM H2)
MM H2 =2 so MM unknown =32
Which is O2.
Under what condition(s) is ΔE = ΔH ?
What is at conditions of constant pressure?
Write an equation (no numbers needed) that represents the total amount of heat absorbed for solid acetone goes to liquid acetone from -120C to -60C.
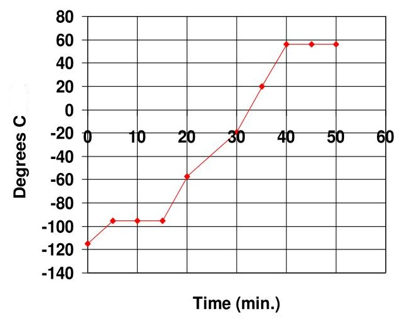
What is q = mcacetone(s) ΔT + n ΔHfus + mcacetone(l)ΔT?
Can get more specific with ΔT (first one is +25, second is +35)
What is Boltzman's constant? (numerical value)
What is 1.38 x 10-23 J/K
What is the orientation factor if the frequency of collisions is 1011 given the Arrhenius plot below?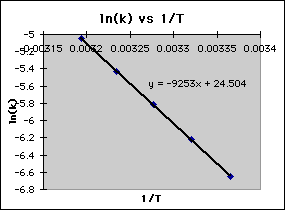
What is 0.438?