Naming Compounds
VSEPR
Lewis Structure
Polarity
100
Which type of organic compound uses this formula:
CnH2n+2
Alkane
100
What does the acronym VSEPR stand for?
Valence Shell Electron Pair Repulsion
100
Name an element that can only hold two electrons
Hydrogen or Helium
100
A molecule's polarity is based on the distribution of what around the atoms?
Electrons
200
What is the correct name for P2O5?
Diphosphorous pentoxide
200
What is the structure of carbon tetrachloride and its bond angle?
Tetrahedral and 109.5 degrees
200
Draw the Lewis dot structure of BH2-
[![]() ]-
]-
200
Would a molecule with symmetric geometry, with only 1 type of molecules around the central atom, be polar or nonpolar?
Polar
300
What is the name of C5H10?
Pentene
300
What is the bond angle of a trigonal planar molecule?
120 degrees
300
Which of the following elements disobeys the octet rule: carbon, fluorine, boron, chlorine
Boron
300
Is this molecule polar?
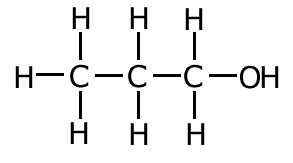
Yes
400
What is the name of this compound? 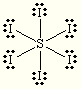

Sulfur hexaiodide
400
What is the molecular geometry and bond angle of N2?
Linear and 180 degrees
400
Draw the Lewis structure of HCN.
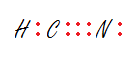
400
Which of the following compounds is nonpolar:
1. 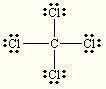 2.
2. ![]() 3.
3. 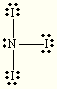
4. 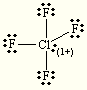
CH4
500
Name this compound:
S2F10
Disulfur decafluoride
500
What is the molecular geometry of PBr5?
Trigonal Bipyramidal, 90 and 120 degrees
500
Draw the Lewis dot structure for ethyne
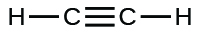
500
Draw the Lewis Structure of NI3 and state its polarity
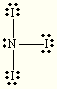 and polar
and polar