The atomic number is the same as the number of this particle.
What is a proton?
This element has 20 protons and 20 neutrons
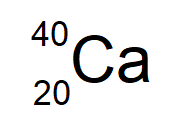
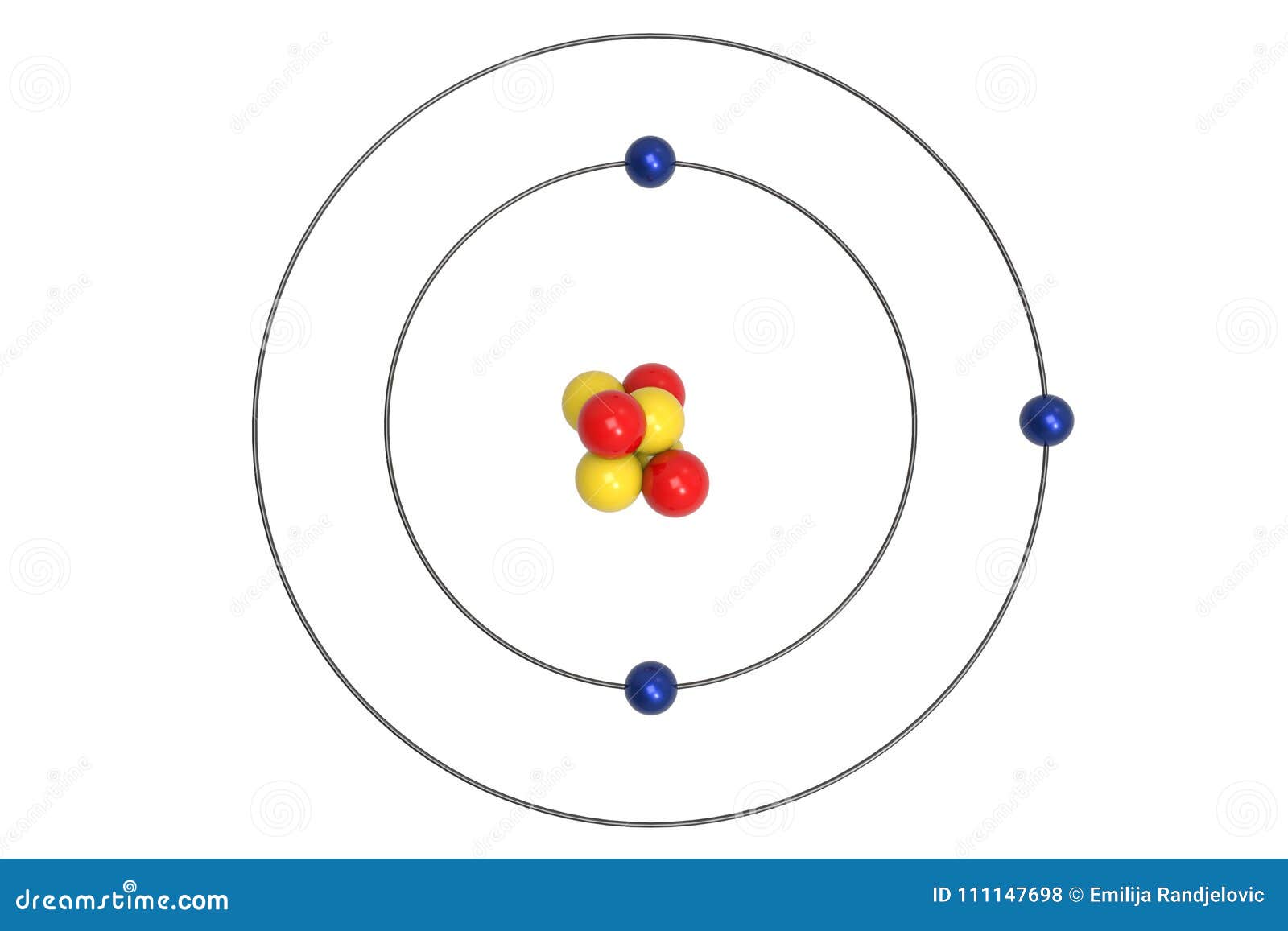
lithium
Number of Neutrons: Beryllium
What is 5 neutrons?
A change in temperature causes a change in this type of energy.
What is thermal energy?
The subatomic particle with no charge and only found in the nucleus.
What is a neutron?
Nitrogen with 8 neutrons
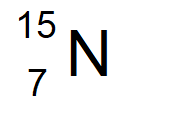
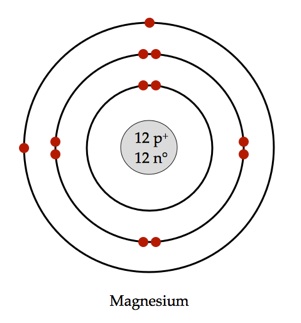
Magnesium
What is 80 protons?
A change from solid to liquid
What is melting?
Found in the nucleus.
What are protons and neutrons
The negatively charged subatomic particle that is found in the orbitals around the nucleus of an atom
What are electrons?
Atomic number 13 and a mass of 28


potassium
Number of electrons in a neutral atom of Cerium (Ce)
What is 58 electrons?
The amount of matter in an object
What is mass?
Have a neutral charge
What are neutrons?
The rings around the nucleus of an atom where electrons stay that make up the electron cloud?
What are the orbitals?
6 Protons and 8 neutrons
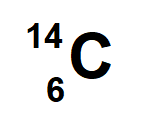
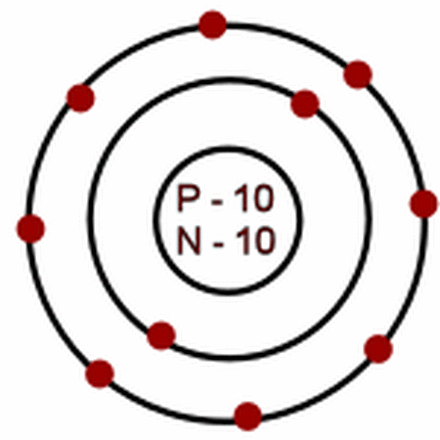
neon
Name of element 50.
What is Tin (Sn)?
A solid to a gas: the process is called
sublimation
The center of the atom where the protons and neutrons stay.
What is the nucleus?
Sodium with a mass of 23 and 1 electron missing
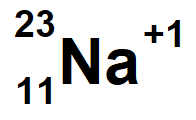
lanthanum
Number of neutrons in Xenon (Xe)?
What is 77 neutrons?
When a tire deflates on a cold night, it is due to what change?
What is a decrease in thermal energy?
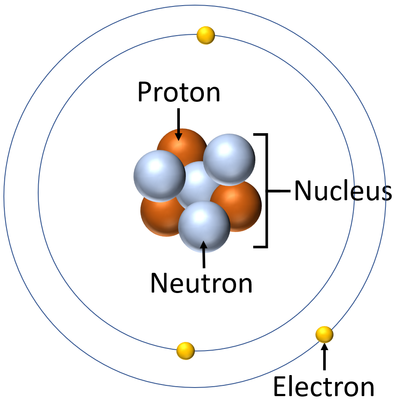
Draw a labeled atom (neutron, proton, electron, nucleus)
The electrons in the orbital that is the farthest away from the nucleus
What are Valence Electrons?
Chlorine with 19 neutrons and an extra electron
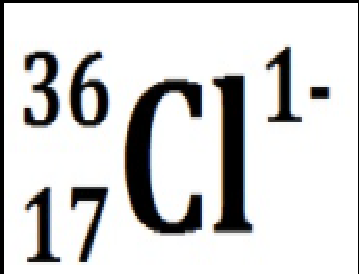
Draw a model of sodium

Every atom of Aluminum has to have the same amount of which subatomic particle?
What are protons?
The two measurements needed to find the speed of an object.
What is distance and time?