This is the process of an atom taking in energy to move up to a higher energy level.
Absorption
What's the density of a 15.00 g rock with 5.0 mL volume?
3.0 g/mL
Which element has the smaller atomic radius?
Li, C, N, F
Fluorine (F)
This element has 28 electrons when neutral.
Nickel (Ni)
This rule states that atoms are most stable when they have a filled valence shell.
The Octet Rule
How much money is made in sales on a 20oC day?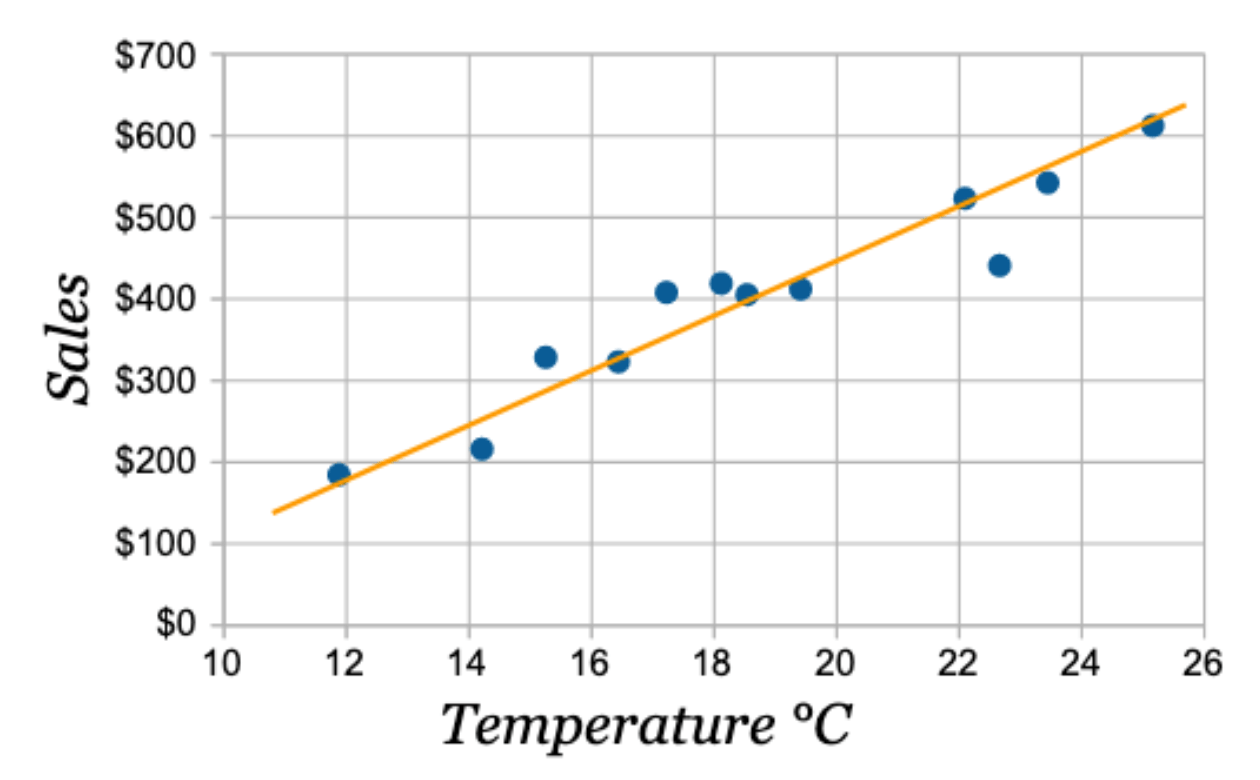
~$450
Which element has the LOWEST ionization energy?
Li, Mg, Rb, Ba
Barium (Ba)
[Kr] 5s2 4d7
Rhodium (Rh)
This rule states that electrons in a sublevel must fill one per orbital before doubling up.
Hund's Rule
Strontium ionizes! Which ELEMENT does is now have the same number of total electrons as?
Krypton (Kr)!
Which element has the LOWEST electronegativity?
K, Fe, Ga, Kr
Krypton (Kr)
This group 4 element has valence electrons in the 5th energy level.
Tin (Sn)
This equation is the subatomic equivalent to Newton's Law of Gravitation and relates distance, charge, and electrostatic force.
Coulomb's Law
The density of iron is 7.84 g/mL. A sample of iron is measured to have a volume of 2.5 mL. What is the mass of the sample?
19.6 g
Which element will have the MOST metallic character?
Al, Cs, Cu, Sr
Cesium (Cs)
When this Period 3 atom loses electrons, it will result in a 3+ charged ion.
Aluminum (Al)
This equation calculates the wavelength of an electronic transition for a Hydrogen atom.
The Rydberg Equation
What's the energy of a transition that results in a wavelength of 435 nm?
4.55 x 10-19 J
Which halogen is MOST reactive?
Fluorine (F)
[Rn] 7s2 5f6
Plutonium, (Pu)