If a product is removed from an equilibrium system at constant temperature, this happens to the position of equilibrium.
What is a shift to the right to produce more product?
The strongest acid among hydrohalic acids due to weakest H–X bond strength.
What is HI?
This is the pOH and pH of a solution with [OH⁻] = 4.50 × 10⁻³ M.
What are pOH = 2.35 and pH = 11.7?
The pH of a 0.100 M NaCl solution.
What is 7.00?
This is the Mission of the Naval Academy.
To develop Midshipmen morally, mentally, and physically and to imbue them with the highest ideals of duty, honor, and loyalty in order to graduate leaders who are dedicated to a career of naval service and have potential for future development in mind and character to assume the highest responsibilities of command, citizenship and government.
This factor speeds up the rate at which equilibrium is reached but does not change the position of equilibrium.
What is a catalyst?
The conjugate base of HSO₄⁻ and the conjugate acid of NH₃.
What are SO₄²⁻ and NH₄⁺?
This is the hydrolysis reaction for methylamine (CH₃NH₂) in water.
What is CH₃NH₂ (aq)+ H₂O (l) ⇌ CH₃NH₃⁺ (aq)+ OH⁻ (aq)?
Whether precipitation will occur when 25.0 mL of 0.010 M AgNO₃ is mixed with 25.0 mL of 0.010 M NaCl (Ksp for AgCl = 1.8 × 10⁻¹⁰).
What is yes, because Q = 2.5 × 10⁻⁷ > Ksp?
[Ag⁺] = 0.00025 mol / 0.050 L = 0.0050 M
[Cl⁻] = 0.00025 mol / 0.050 L = 0.0050 M
Q = [Ag⁺][Cl⁻] = (0.0050 M)(0.0050 M) = 2.5×10⁻⁵
This is the standard-issue close-in weapon system (CIWS) used to defend Navy ships against anti-ship missiles is affectionately nicknamed this.
What is the Phalanx or "R2-D2"?
According to Le Châtelier’s Principle, this happens to the equilibrium position of a gaseous system when the volume is decreased.
What is a shift toward the side with fewer moles of gas?
This acid is the weakest in water. 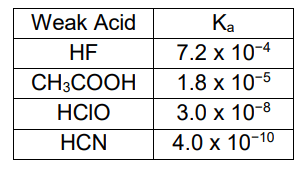
What is HCN?
Consider the following titration curve of HNO2 with NaOH. At this point in the titration, [HNO2]=[NO2−].
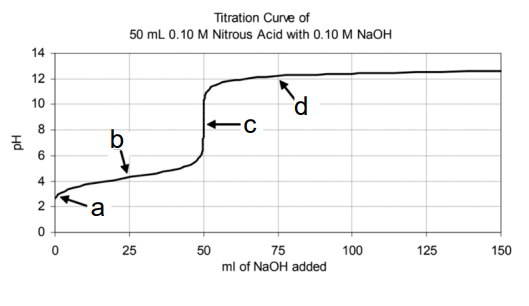
What is point C.
This is the molar solubility of PbI₂ in 0.10 M NaI (Ksp = 8.5 × 10⁻⁹).
What is approximately 8.5 × 10⁻⁷ M?
PbI₂(s) ⇌ Pb²⁺(aq) + 2 I⁻(aq)
Ksp = [Pb²⁺][I⁻]² = s (0.10)²
s = (8.5 × 10⁻⁹) / (0.01) = 8.5 × 10⁻⁷ M
What time is it?
Sir, I am greatly embarrassed and deeply humiliated that due to unforeseen circumstances beyond my control, the inner working and hidden mechanisms of my chronometer are in such inaccord with the great sidereal movement with which time is generally reckoned that I cannot with any degree of accuracy state the correct time, Sir. But without fear of being to greatly in error, I will state that it is about __minutes, __seconds, __ticks past __bells.
For the endothermic reaction N₂ + 3H₂ ⇌ 2NH₃, this is the effect of increasing temperature.
What is a shift toward the products (more NH₃ is formed)?
The second ionization step of sulfuric acid in water.
What is HSO₄⁻ → H⁺ + SO₄^2⁻ ?
This is the Kb of a weak base if a 0.15 M solution has a pH of 10.95.
What is approximately 5.3 × 10⁻⁶?
B + H₂O ⇌ BH⁺ + OH⁻
pOH = 14 - 10.95 = 3.05
[OH⁻] = 10^(–pOH) = 10^(–3.05) ≈ 8.91 × 10⁻⁴ M
x = [OH⁻] ≈ 8.91 × 10⁻⁴ M
At equilibrium: [B] ≈ 0.15 – x ≈ 0.15 M and [BH⁺] ≈ x
Kb = ([BH⁺][OH⁻]) / [B]
Kb ≈ (8.91 × 10⁻⁴)² / 0.15
(8.91 × 10⁻⁴)² ≈ 7.94 × 10⁻⁷
Kb ≈ (7.94 × 10⁻⁷) / 0.15 ≈ 5.29 × 10⁻⁶
This is the pH in the titration of 50.0 mL of 0.120 M acetic acid by 0.240 M sodium hydroxide after the addition of 35.0 mL of base.
What is pH=12.5?
Acetic acid: 50.0 mL × 0.120 M = 0.0060 mol
NaOH: 35.0 mL × 0.240 M = 0.0084 mol
Excess OH = 0.0084 – 0.0060 = 0.0024 mol
Total volume = 50.0 mL + 35.0 mL = 0.085 L
[OH⁻] = 0.0024 mol / 0.085 L ≈ 0.02824 M
pOH = –log(0.02824) ≈ 1.55
pH = 14 – 1.55 ≈ 12.45
Capable of carrying Trident II D5 missiles, this class of submarine serves as the sea-based leg of the nuclear triad.
What is the Ohio-class submarine?
A 1.00 L container initially contains 0.400 mol N₂ and 1.20 mol H₂. At equilibrium, 0.100 mol NH₃ is present. This is the value of K.
N2+3H2⇌2NH3
What is K = 0.0247?
Producing 0.100 mol NH₃ means 0.050 mol N₂ and 0.150 mol H₂ are consumed. This gives equilibrium amounts of 0.350 mol N₂, 1.05 mol H₂, and 0.100 mol NH₃ in a 1.00 L container (so the concentrations are 0.350 M, 1.05 M, and 0.100 M, respectively). The equilibrium constant is K = [NH₃]² / ([N₂][H₂]³) = (0.100)² / (0.350 × (1.05)³) ≈ 0.0247.
This is the structural reason why HNO₃ is a stronger acid than HNO₂.
What is greater resonance stabilization of the conjugate base?
The pH of a buffer made by mixing 0.15 mol NH₃ and 0.10 mol NH₄Cl in 1.00 L (Kb = 1.8 × 10⁻⁵).
What is approximately 9.44?
pKb = –log(1.8×10⁻⁵) ≈ 4.74
pKa = 14 – pKb ≈ 14 – 4.74 = 9.26
pH = 9.26 + log(0.15/0.10)
pH = 9.26 + log(1.5)
pH ≈ 9.26 + 0.176 = 9.44
This is the pH of a solution made by mixing 0.10 M CH₃COOH and 0.10 M CH₃COONa (Ka = 1.8 × 10⁻⁵).
What is pH = 4.74?
Why Didn't you say "sir"?
Sir, "sir" is a subservient word surviving from the surly days of old Serbia, when certain serfs, to ignorant to remember their lord's names, yet to servile to blaspheme them, circumvented the situation by surrogating the subserviant word "sir," by which I now belatedly address a certain senior cirriped, who correctly surmised that I was syrupy enough to say "sir" after every word I said, Sir.
In the reaction PCl₅ (g) ⇌ PCl₃ (g) + Cl₂ (g) , a decrease in volume affect the equilibrium position in this direction.
What is a shift towards PCl₅ (left)?
This is the pH of a buffer that contains 0.12 M lactic acid (HC₃H₅O₃) and 0.10 M sodium lactate (NaC₃H₅O₃). The acid dissociation constant is Kₐ = 1.4×10⁻⁴.
What is a pH of 3.77?
Kₐ = 1.4×10⁻⁴, so pKₐ = –log(1.4×10⁻⁴) ≈ 3.85.
Using the Henderson–Hasselbalch equation:
pH = 3.85 + log(0.10/0.12)
pH = 3.85 + log(0.8333)
pH ≈ 3.85 – 0.079 ≈ 3.77
This is the pH at the equivalence point for titrating 0.100 M NH₃ with 0.100 M HCl. Kₐ = 5.56×10⁻¹⁰
What is approximately 5.28?
[NH₄⁺] = (0.100 mol/L × 2L)= 0.050 M.
Kₐ ≈ x² / 0.050→x² = Kₐ × 0.050→x² ≈ (5.56×10⁻¹⁰)(0.050) = 2.78×10⁻¹¹
x ≈ √(2.78×10⁻¹¹) ≈ 5.27×10⁻⁶ M.
pH = –log(5.27×10⁻⁶) ≈ 5.28.
In a saturated solution of Ca(OH)2(s) at equilibrium, the concentration of calcium ions is 0.015 M and the concentration of hydroxide ions is 0.030 M. This is the predicted value of Ksp for Ca(OH)2(s).
What is 1.4 x 10−5?
Ca(OH)₂(s) ⇌ Ca²⁺(aq) + 2 OH⁻(aq)
Ksp = [Ca²⁺] · [OH⁻]²
[Ca²⁺] = 0.015 M
[OH⁻] = 0.030 M
Ksp = (0.015) · (0.030)²
= 0.015 · 0.0009
= 1.4 × 10⁻⁵
This term refers to the U.S. Navy’s ability to control and operate freely in contested waters.
What is sea control?
These are the final equilibrium concentrations of all species when 2.00 mol COCl₂ is placed in a 5.00 L container and allowed to reach equilibrium with K = 1.2 × 10⁻².
COCl 2 (g)⇌CO(g)+Cl 2 (g)
What are [CO] = [Cl₂] ≈ 0.0636 M, [COCl₂] ≈ 0.400–0.0636 = 0.336 M?
Initially, the concentration of COCl₂ is 2.00 mol/5.00 L = 0.400 M, with CO and Cl₂ at 0 M.
At equilibrium: [COCl₂] = 0.400 – x [CO] = x [Cl₂] = x
The equilibrium constant is given by:
K = [CO][Cl₂] / [COCl₂] = x²/(0.400 – x) = 1.2×10⁻²
Solving for x: x² = 1.2×10⁻² (0.400 – x)
x ≈ 0.0636 M
This is the pH at the half-equivalence point of a titration of 50.0 mL of 0.10 M HF with 0.10 M NaOH (Ka = 6.6 × 10⁻⁴).
What is 3.18?
At the half-equivalence point of a weak acid titration, the concentration of the acid equals the concentration of its conjugate base, so the pH equals the pKa.
For HF, pKa = –log(6.6×10⁻⁴) ≈ 3.18.
A 1.0 L solution contains 0.060 moles of LiF and 0.040 moles of HF (pKa = 3.17). This is the pH after 0.010 moles of HCl have been added, neglecting changes in volume.
What is pH = 3.17?
New amounts:
HF: 0.040 + 0.010 = 0.050 mol
F⁻: 0.060 − 0.010 = 0.050 mol
Since the solution volume remains 1.0 L, the concentrations are:
[HF] = 0.050 M [F⁻] = 0.050 M
pH = 3.17 + log(0.050/0.050)
pH = 3.17 + log(1) pH = 3.17
Describe the acidic/basic character of each of the following salts:
a). LiF
b) NH₄Cl
c). NaCH₃COO
d). Na₂CO₃
e). KCN
f). (NH₄)₂SO₄
a). Basic
b). Acidic
c). Basic
d). Basic
e). Basic
f). Acidic
You are an ensign on a Virginia class submarine actively engaging in combat. Uniquely enough, your enemy combatant is allergic to the sight of chemistry titration curves. You are so thankful that you have been rigorously trained in plebe chemistry, your favorite class at the Naval Academy. In order to neutralize the threat, you must draw two titration curves. One featuring a strong acid vs. strong base, the other one featuring a weak acid vs. strong base. If applicable, you must label where pH = pKa, circle the buffer zone, and indicate when moles of base exceed moles of acid.
The effect on the position of equilibrium when inert gas is added to a constant-volume system.
What is no shift in equilibrium position?
This is the pH of a solution containing 0.100 M of a weak monoprotic acid with 2.0% ionization.
What is pH ≈ 2.7?
The acid is 2.0% ionized, so the [H⁺] produced is 2.0% of 0.100 M, which is 0.0020 M.
pH = –log[H⁺]
pH = –log(0.0020)
pH ≈ 2.70
This is the value of Kb for a weak base if a 0.050 M solution has a pOH of 4.80.
What is Kb ≈ 5.0×10⁻⁹?
At pOH = 4.80, the [OH⁻] is 10^(–4.80) ≈ 1.58×10⁻⁵ M.
Kb = (x²) / (0.050)
Kb ≈ (1.58×10⁻⁵)² / 0.050
≈ 2.50×10⁻¹⁰ / 0.050
≈ 5.0×10⁻⁹
The delta G° value for a reaction is +36.38 kJ/mol. This is the equilibrium constant for the reaction at 25°C.
What is 4.2 × 10⁻⁷?
ΔG° = –RT ln K
ΔG° = +36.38 kJ/mol = 36380 J/mol,
R = 8.314 J/(mol·K)
ln K = –(36380)/(8.314 × 298)
= –(36380)/(2478.7)
≈ –14.68
K = e^(–14.68) ≈ 4.2 × 10⁻⁷.
This is an example of a complete chow call.
Answer will depend upon satisfactory chow call as determined by the class.