How many protons, neutrons and electrons are there in an atom of 197Au
The superscript represents the mass number (protons + neutrons)
197Au has 79 protons, 79 electrons, and 197 -79 = 118 neutrons
HCl + Na₂S → what type of reaction?
Double Displacement
CO2 + 2LiOH --> Li2CO3 + H2O How many moles of lithium hydroxide are required to react with 20 mol of CO2?
40 moles
The substance in which the solute dissolves
Solvent
What is the mathematical formula for Charles's Law
V1/T1=V2/T2
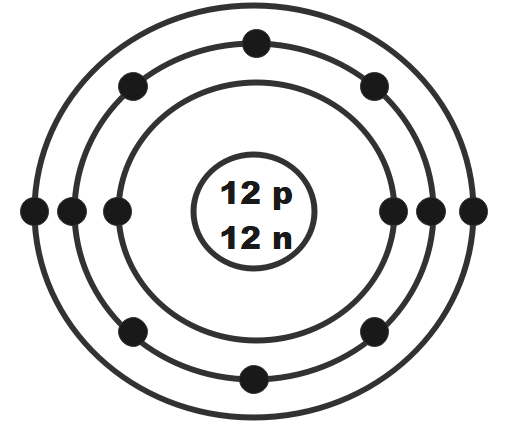
Draw the Bohr-Rutherford diagram for magnesium
Br₂ + NaCl -->Result?
No Reaction
The measured amount of product obtained after performing a chemical reaction.
Actual Yield
Name 1 way that you can increase the rate of solubility of any substance.
increase temperature
increase movement
increase surface area
The temperature at which the motion of particles theoretically ceases
0 Kelvin or -273 degrees C
What is the formula of: hydrochloric acid, chloric acid and chlorous acid
HCl, HClO3, HClO2
Balance: NH3 + CuO --> Cu + N2 + H2O
2 NH3 + 3 CuO --> 3 Cu + N2 + 3 H2O
How many moles of aluminum atoms are needed to combine with 1.58 mol of oxygen molecules to make aluminum oxide?
2.1 moles of Al
If 1,000 grams of BaCO3 are dissolved to make a 2.2 molar solution, what must be the minimum volume of the container?
2.3 L
if a sample of gas occupies 6.80L at 325 deg C, what will its volume be at 25 deg C if the pressure does not change?
8.36 L
Draw the Lewis Diagrams of H3O+

Difference between complete and incomplete combustion?
Products for complete combustion: CO2 and H2O
Products for incomplete combustion: CO2 and H2O and CO (and C)
Sodium hydroxide reacts with sulfuric acid (H2SO4; MM=98.1 g/mol) to form water and sodium sulfate. How many grams of sodium sulfate (MM=124 g/mol) will be formed from 200 g NaOH (MM=40 g/mol) and an excess of sulfuric acid?
355 g
Calculate the mass of BaSO4 formed when 0.875L of .200M Na2SO4 solution is added to sufficient BaCl2 solution.
40.8 g
A gas at 155kPa and 25 deg C has an initial volume of 1.00L. The pressure of the gas increases to 605 kPa as the temperature is raised to 125 deg. C. What is the new volume?
0.34 L
Provide the name of: HBr, HC2H3O2, H2SO3
hydrobromic acid
acetic acid
sulfurous acid
Balance: ___ FeS2 + ___ O2 --> ___ Fe2O3 + ___ SO2
4 FeS2 + 11 O2 --> 2 Fe2O3 + 8 SO2
This questions is worth 1000 points! SURPRISE! Chlorine gas reacts with potassium bromide to produce potassium chloride and bromine gas. How many grams of potassium chloride can be produced from 300. g each of chlorine and potassium bromide?
188 g
How many liters of 0.150M KI would be required to react completely with 15.00 grams of lead (II) nitrate?
0.604 L
A sample of hydrogen has a volume of 8.56L at 0 deg C and 1.5 atm. Calculate the number of molesof hydrogen present in the sample?
0.57 moles