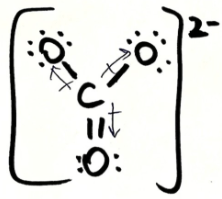
Is CO3 2- polar or non-polar? Explain your answer. If it is polar, draw the vectors and label the δ+ and δ- end
non-polar
Is BrCl5 ionic, polar covalent or non-polar covalent? Explain your answer. If it is polar, draw the vectors and label the δ+ and δ- end
polar covalent 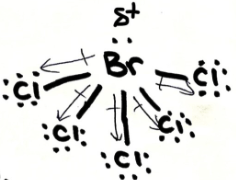
Is AlF3 ionic, polar covalent or non-polar covalent? Explain your answer. If it is polar, draw the vectors and label the δ+ and δ- end
ionic 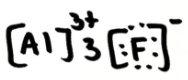
Which structure shows Iron (II) Oxide?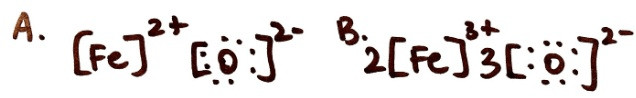
A
Which is the most reactive non-metal and WHY?
F because it has the smallest atomic radius (aside from He - which is an inert gas).
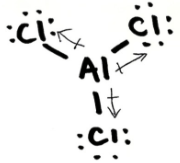
Is AlCl3 ionic, polar covalent or non-polar covalent? Explain your answer. If it is polar, draw the vectors and label the δ+ and δ- end
non-polar due to vectors cancelling out
Is HF ionic, polar covalent or non-polar covalent? Explain your answer. If it is polar, draw the vectors and label the δ+ and δ- end
ionic 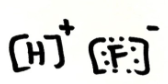
Rank the following in order of LARGEST to SMALLEST atomic radii: Ti, Ra, Ca
Ra > Ca > Ti
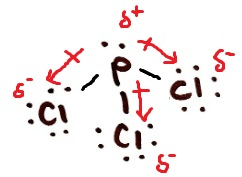
Draw and classify phosphorus trichloride as ionic, polar covalent, or non-polar covalent. Draw all dipoles and label any δ+ and δ- end if applicable
Is NO3 - polar or non-polar? Explain your answer. If it is polar, draw the vectors and label the δ+ and δ- end
non-polar due to vectors cancelling out 
Draw the Lewis Structure for SO3 2- and classify it as polar or non-polar. If it is polar, draw the vectors and label the δ+ and δ- end
polar 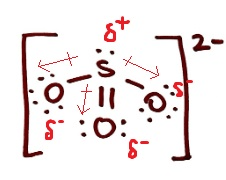
Draw the Lewis Structure for acetate ion and classify it as polar or non-polar. If it is polar, draw the vectors and label the δ+ and δ- end
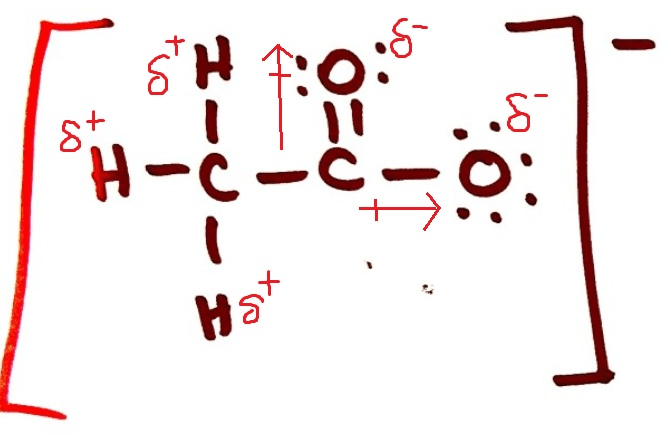
Rank the following in order of MOST soluble in water to LEAST soluble: Ni (s), BrF, BrCl5, C2H6 , Copper (II) sulfide
CuS > BrF > BrCl5 > C2H6 > Ni (s)
What intermolecular forces does SO3 2- have in water? Draw and list them
H-bonding, ion-dipole, and LDFs
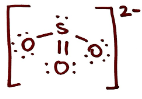
Rank the following in order of LEAST reactive to MOST reactive: Si, Cl, S, Ne, F
Ne < Si < S < Cl < F
What intermolecular forces does AlF3 have in alcohol like CH3OH? Draw and list them.
ion-dipole and LDFs 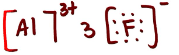
Rank these structures for CO3 2- from most stable to least stable 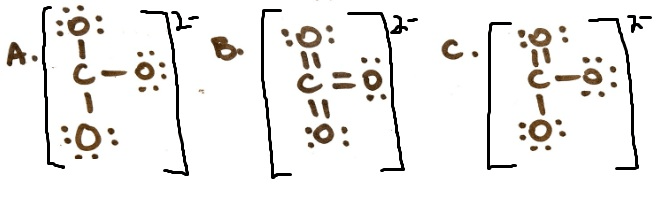
C, B, A