____________ said that electrons exist in energy packets called quanta
Planck
Draw and name the interaction that occurs between sulfur dioxide and He
Dipole-induced dipole interaction
_____________ did an experiment to prove the atom was mostly empty space and there is a positive center (the nucleus)
rank the following in order of LEAST to MOST soluble in oil:
NaCl, NH3, C3H8
NaCl < NH3 < C3H8
_____________ organized elements according to atomic mass
Mendeleev
Is CCl2O polar or non-polar? Explain your answer. If it is polar, draw the vectors and label the δ+ and δ- end
non-polar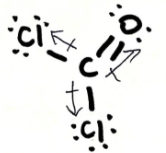
Does AlF3 dissolve well in alcohols? explain
yes because it is ionic and alcohols are polar (ion-dipole interactions)
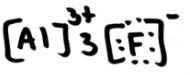
rank the following in order of LEAST to MOST soluble in water:
PCl5, NaNO3, SO2, Fe (s)
Fe (s) < PCL5 < SO2 < NaNO3
 Which is the correct Lewis structure for CO and WHY?
Which is the correct Lewis structure for CO and WHY?
does CO dissolve in water easily? explain
yes because it is polar and therefore H-bonds (dipole-dipole)

Rank the following in order of MOST to LEAST soluble in methane (CH4):
C2H2, HF, Cu (s), CO2
C2H2 > CO2 > HF > Cu (s)
Does SiBr4 dissolve well in oil? Explain
yes because it is non-polar and ''like dissolves like''
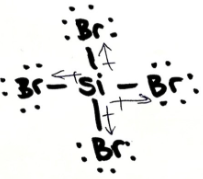
Write the chemical formula for each Lewis structure
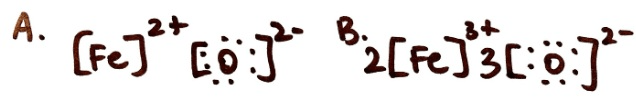
Is SO4 2- polar or non-polar? Explain your answer. If it is polar, draw the vectors and label the δ+ and δ- end
non-polar 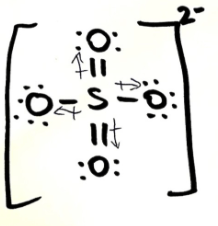
calculate the average atomic mass of the germanium isotopes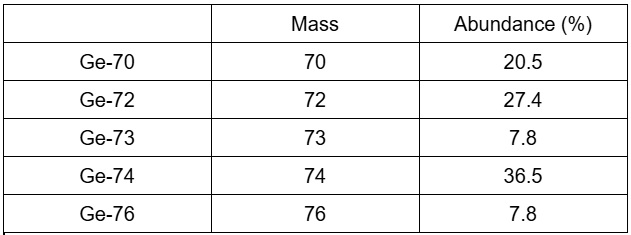
72.71 amu
Which structure for acetate is the most stable and why?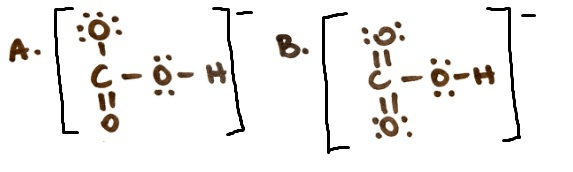
A because the - charge is on the O instead of the C
The average atomic mass of rhenium is 186.21 amu. What is the mass of Re-187?
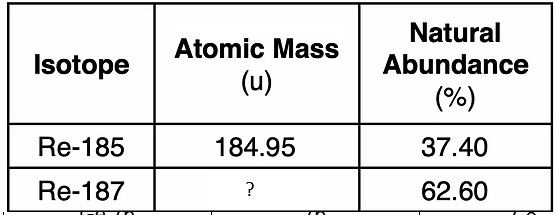
186.96 amu
What kind of intermolecular forces occur between molecules of CH4?
only LDFs
List the following in order of HIGHEST to LOWEST boiling point:
solid copper, CO, HCl, CCl2O
solid copper > HCl > CO > CCl2O
List 2 differences between a photon and an electron
* photon has no charge and electron is negative
* photon has no mass but electrons have a mass
* photons make up the electromagnetic spectrum and electrons make up atoms