What holds ionic bonds together?
Formula for: magnesium perchlorate
Mg(ClO4)2
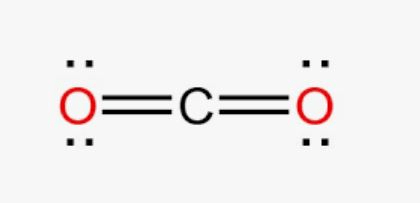
Draw the bonding structure for CO2
True or False: nonpolar molecules dissolve in water
False
"Like dissolves like"
What holds a covalent bond together?
Which conducts electricity better, NaCl or HCl?
Why?
NaCl - free-to-move charges
Name for: Re(AsO3)2
Rhenium (VI) arsenite
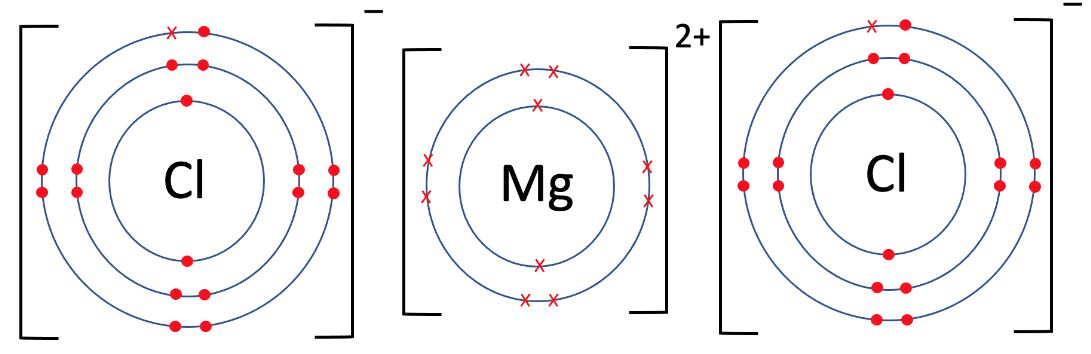
Draw the bonding structure for MgCl2
True or False: a polar molecule must be asymmetric
True
Explain the concept of polarity
Electrons spend more time with one atom than another
_______________ compounds can be soluble or non-soluble in water.
Molecular
Name and bond polarity for: P2S3
diphosphorus trisulfide
bond: non-polar
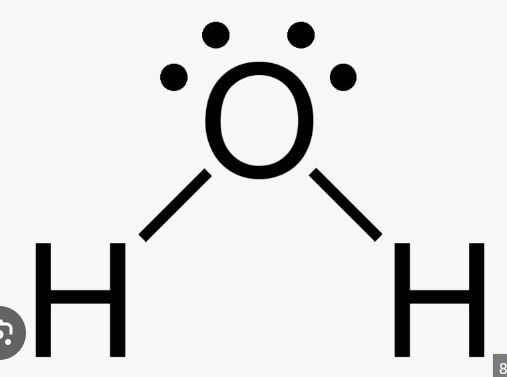
Draw the bonding structure for H2O. Determine the bond polarity and the molecular polarity.
Bond polarity = polar
Molecular polarity = polar
Calculate the △EN and determine the type of bond for nitrogen monoxide
△EN = 0.4 ∴ this is a non-polar bond
What is electronegativity used to determine?
the extent of electron sharing in a bond
Explain why ionic solids dissolve in water
ion-dipole forces
Water is a polar substance. The cation of the ionic solid is attracted to the negative end of the water and the anion is attracted to the positive end of water.
Name for: H2CrO4
chromic acid
First determine the
triangleEN ,
then draw the appropriate bonding structure for AlCl3
triangle EN = 1.55
therefore, polar covalent
Determine the bond polarity of : CH3Br
Determine the molecular polarity of the molecule
Bond: non-polar (C-H) and polar (C-Br)
Molecule: polar (asymmetrical)
What previously held understanding about ionic and molecular compounds, have been proven incorrect based on our understanding of electronegativities?
Previous knowledge - metal + non-metal = ionic, non-metals = covalent
New knowledge = difference of EN has to be greater than 1.7 in order for an ionic compound to be formed
Which compound will have the higher boiling point, MgO or H2O? Why.
MgO - strong bonds between ionic solids
Formula for: hypochlorous acid
*Fun fact! This is a substance that our bodies naturally produce within our white blood cells, it works to kill bacteria and irritants that are harmful to us!
HClO (aq)
Determine the type of bond, then draw the bonding structure for PCl3
Bond = polar covalent
Determine the bond polarity of CCl4
Determine the molecule polarity
Bond: polar
Molecule: non-polar