The atomic number of iron
26
In this state of matter, atoms or molecules cannot move freely but they vibrate in place.
Solid
This is the positively charged particle in an atom
proton
Number of protons

40
MgCl2
magnesium chloride
H2O
Water
The elemental symbol for potassium
This type of substance does not have its own shape and can be compressed.
Gas
This particle is not part of the nucleus of an atom
electron
Atomic number

42
SiO2
silicon dioxide
NaCl
salt/ table salt
What element is diamond made of?
Carbon
Turning a solid into a liquid is called
Melting/ Fusion
This particle is what is gained or lost to form ions
electron
Mass number
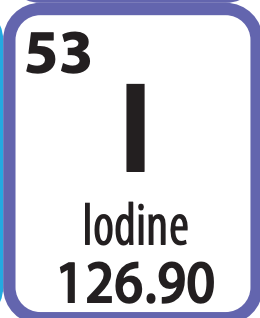
127
or 126.90
CuCl3
copper (iii) chloride
CH3COOH
These elements are highly reactive and contain 7 valence electrons
Halogens
Turning a liquid into a gas is called
Evaporation
These two subatomic particles contribute to the mass of an atom
protons and neutrons
Number of electrons (in a neutral atom)
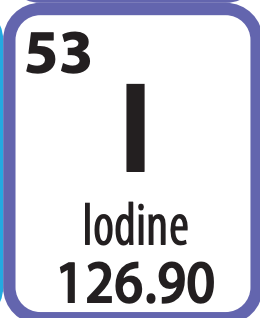
53
Daily Double
What is the chemical formula for iron (iii) carbonate?
C12H22O11
sugar (sucrose)
This latin name for gold is where the chemical symbol for gold comes from
When a solid sublimates, it becomes this state of matter.
gas
This type of elementary particle makes up protons and neutrons
quarks
Number of neutrons

10
K2Cr2O7
Potassium dichromate
NaHCO3
baking soda (sodium bicarbonate)