What family of elements is the least reactive?
Noble Gases
What is the chemical name of this compound: Fe2S3
Iron (III) Sulfide
Energy that is stored is called
Potential Energy
Nuclear Fusion is:
Where does it occur, and what atoms does it most commonly use?
The combination of two small nuclei into one larger nucleus.
In stars, hydrogen
What are the three components of a Nucleotide
Phosphate, Sugar and Nitrogenous Base
How many valence electrons does sulfur have?
6 valence electrons
What is the chemical name of this compound: H2O
Dihydrogen monoxide
A vacuum sealed, insulated container like a thermos is considered what kind of system?
Nuclear Fission is:
what does it release? (2)
The splitting of one large nucleus into two smaller nuclei.
Releases neutrons and energy
Name all base pairs.
Which ones bind together?
Adenine - Thymine
Guanine - Cytosine
A covalent bond is between _________ and ________ where the electrons are _________
Non-metal and Non-metal
shared
Name the following compound: K2CO3
Potassium Carbonate
Atomic Number refers to: _________
Mass Number refers to: _________
Number of Protons
Number of Protons + Neutrons
When an Exothermic reaction occurs, the energy of the products is greater/less than the energy of the reactants
Less than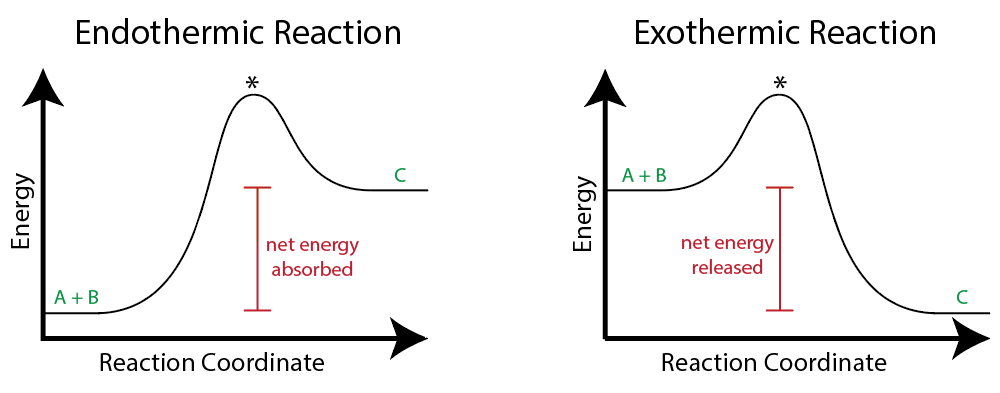
A flower that has white pedals is crossed with a flower with red pedals. The offspring expresses red and white pedals. This is an example of: ______________
Codominance
An ionic bond is between _________ and ________ where the electrons are _________
Metal and Non-metal, transferred
What is the formula for Copper (II) Oxide
CuO
Determine the potential energy of a 1.29kg rock that is raised 5.3m.
67 Joules
What is the product of Uranium-235 that emits an alpha particle
Thorium-231
23190Th
A species of bird migrates to a newly formed island, and over thousands of years, becomes 20 distinct species. This is an example of:
Adaptive Radiation
An acid typically starts with this ion: ___
A base typically ends with this ion: ___
Acid: H+
Base: OH-
Predict and Balance the following reaction:
__ K + __ MgO --> ?
2 K + __MgO --> __ Mg + __ K2O
An 800kg polarbear runs 9m/s
A 65kg cheetah runs 30m/s
What is the kinetic energy of both animals, and which one has more kinetic energy?
Polar Bear: 32,400 J
Cheetah: 29,250 J
What is the product of Thorium-232 that emits a Beta Particle
Protactinium-232
23291Pa
Green (G) is dominant to Yellow (g) in Peas. What is the genotype and phenotype of the possible offspring when two heterozygous green peas are crossed?
Heterozygous (Gg) x (Gg)
Genotype: GG = 25%, Gg = 50%, gg = 25%
Phenotype: Green = 75%, Yellow = 25%