Which process results in the formation of water on the outside of a cold glass of iced tea on a warm day?
(1) boiling
(2) condensation
(3) freezing
(4) evaporation
2 Condensation
Which change is the best example of a physical change?
(1) a cookie baking
(2) paper burning
(3) ice cream melting
(4) a nail rusting
ice cream melting
How are the particles arranged in a solid?
A. Packed together tightly
B. Spread out
C. Packed together loosely
Packed together tightly
A student placed a rock in a graduated cylinder containing water, causing the water level in the cylinder to increase by 20 mL. This increase represents the rock’s
(1) mass
(2) solubility
(3) volume
(4) temperature
Volume
The diagram represents a rock that was placed in a graduated cylinder containing 20 mL of water, causing the water level to rise.
Which physical property of the rock is being measured using the graduated cylinder?
(1) volume (2) solubility
(3) mass (4) hardness
1.) Volume
The diagram represents three phases of matter, labeled A, B, and C.
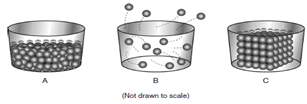 Which table correctly identifies the phases of matter represented by the diagrams?
Which table correctly identifies the phases of matter represented by the diagrams?
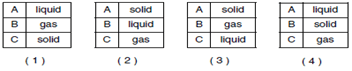
A Liquid
B Gas
C Solid
Which activity involves only a physical change?
(1) grinding coffee beans
(2) baking cookies
(3) acid bubbling on rock
(4) exploding fireworks
Grinding coffee beans
How are the particles arranged in a gas?
Spread out.
If i have an object that has a mass of 10 grams and a volume of 2 ml. What is the density?
5 g/ml
One example of matter is
(1) magnetism
(2) water
(3) heat
(4) radiation
Water
What phase change takes place during melting?
Solid to Liquid
Hydrochloric acid is added to a beaker containing a piece of zinc. As a result, zinc chloride is formed and hydrogen gas is released. This is an example of
(1) a chemical reaction
(3) photosynthesis
(2) a physical change
(4) evaporation
Chemical Reaction
A substance in the gas phase has
A. Definite Shape and Definite Volume
B. Definite Shape and Indefinite Volume
C. Indefinite Shape and Definite Volume
D. Indefinite Shape and Indefinite Volume
D- Indefinite Shape and Indefinite Volume
Which property of the liquid can be directly measured using the graduated cylinder?
(1) mass
(2) solubility
(3) density
(4) volume
Volume
A substance in the solid phase (state) of matter has
(1) a definite shape and a definite volume
(2) a definite shape, but no definite volume
(3) no definite shape, but a definite volume
(4) no definite shape and no definite volume
Definite shape and a definite volume
The change from a gas to a liquid is called
What type of change occurs when a nail rusts?
Chemical Change
What happens when a liquid is heated to it's boiling point?
It evaporates or turns to a gas.
Which object is more dense?
A- Mass of 10 grams and volume of 2 ml.
B- Mass of 10 grams and volume of 1 ml.
C- Mass of 8 grams and a volume of 2 ml.
D- Mass of 12 grams and a volume of 2 ml.
B- Mass of 10 grams and a volume of 1 ml.
What are the three states of matter?
Solid, Liquid, Gas
What do we call the change from a liquid to a gas?
Evaporation
What phase change occurs when an egg is fried?
Chemical
How can I go from a solid to a gas? Remember- It skips a step and it is rare.
Sublimation
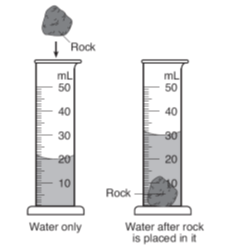 The diagram shows a rock being placed into a graduated cylinder. What is it's volume?
The diagram shows a rock being placed into a graduated cylinder. What is it's volume?
10 ml
A substance in the liquid phase has
A. Definite Shape and Definite Volume
B. Definite Shape and Indefinite Volume
C. Indefinite Shape and Definite Volume
D. Indefinite Shape and Indefinite Volume
C- Indefinite Shape and Definite Volume