Useful Chemistry
Useful Chemistry
Common Reactions in our Lives
Types of Chemical Reactions
Reactions and the Environment
What does it mean if something is made of synthetic polymers?
It is made out of a material that is not natural.
Name a chemic al reaction that you encounter on a daily basis.
Answers may vary
I just ate some spicy food and I have heartburn and acid reflux. I took some Tums to counteract the acid in my stomach. What type of reaction am I relying on to make me feel better?
A neutralization reaction.
What type of reaction is the burning of fossil fuels?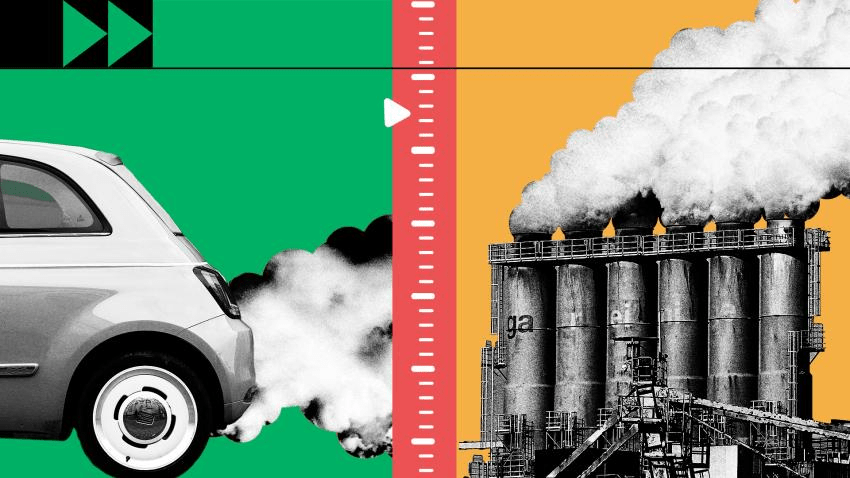
A combustion reaction.
Before synthetic fibres, what were our clothes mostly made of?
Cotton, wool, and other natural fibres.
What is an alloy?
A metallic substance composed of two or more elements.
List 2 indicators of chemical change.
increase or decrease in temp
production of gases (bubbles)
formation of solids (precipitate)
colour change
odours given off
change in pH
What type of reaction is this?
SnI → I2 + Sn
This is a decomposition reaction.
What is the main thing that makes incomplete combustion dangerous?
The production of carbon monoxide.
List 3 elements.
Answers will vary. 
Synthetic chemicals are human-made chemicals produced in a lab. Name two types of synthetic chemicals that a farmer may use.
Some examples of synthetic chemicals used for farming include:
herbicides
insecticides
fungicides
larvacides
What is an endothermic reaction?
Reactions that absorb energy.
What type of reaction is this?
C6H12O6 + O2 → CO2 + H2O + energy
This is a combustion reaction.
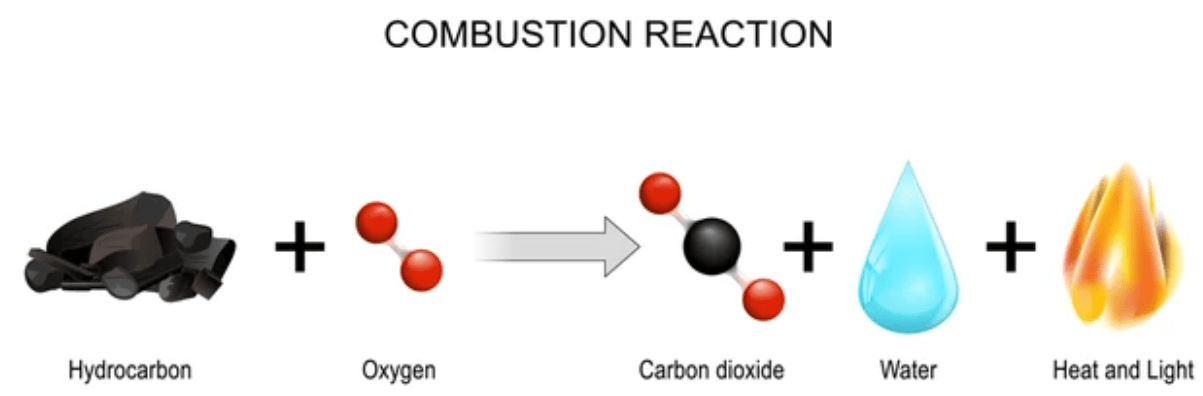
How would a bridge maintenance crew slow the rusting of a bridge over many years?
By painting it as it rusts.
Explain the Greenhouse effect to me like I'm 5 years old.
The greenhouse effect is like a warm blanket around the Earth. It happens because certain gases in the air keep the Sun's warmth from escaping back into space. This makes our planet just the right temperature for us to live on.
What is the system of symbols put in place to prevent workplace accidents involving chemicals?
WHMIS
What is an exothermic reaction?
Reactions that release energy.
What type of reaction is this?
Fe + O2 → Fe2O3
This is a formation reaction.
DOUBLE Jeopardy: What is it called when calcium carbonate is dropped into a lake to neutralize the lakes pH?
Liming.
List 2 negative consequences of climate change.
Answers will vary.
DOUBLE Jeopardy: In any given chemical reaction, two or more initial substances react to produce two or more new substances. What are the initial substances known as? 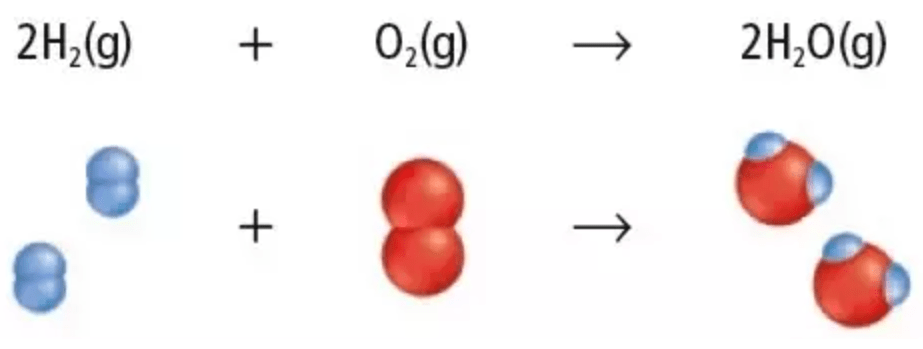
Reactants
Name the 4 types of reactions discussed in this chapter.
Combustion
Neutralization
Composition
Decomposition
What is the Law of Conservation of Mass?
The Law of Conservation of Mass states that matter cannot be created nor destroyed; rather, it is transformed from one form to another.
Why do we need healthy forests to combat climate change?
Healthy forests convert harmful carbon dioxide back into Oxygen and store carbon in it's trunk and branches.
FINAL Jeopardy:
To reduce emissions, the component removed from natural gas before burning is ___________.
Sulfur.