What tiny negatively charged particle moves around the nucleus of an atom?
Electron
What do we call the one‑ or two‑letter that represents a chemical element on the periodic table?
ex. Na, Al,
Element symbol
Which positively charged particle is found in the nucleus of an atom?
Proton
What do we call a pure substance made of only one type of atom?
Chemical element
Which particle in the nucleus of an atom has no electric charge?
Neutron
What do we call a horizontal row on the periodic table?
Period
What are they?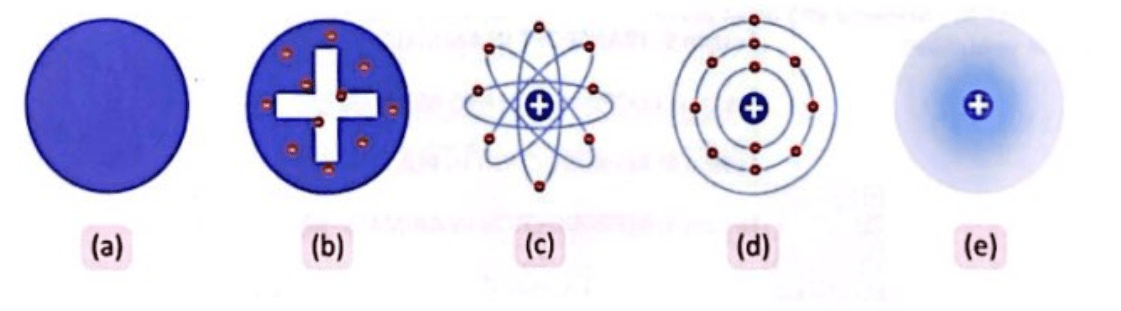
Atomic models
What type of element is usually shiny, can conduct heat and electricity, and is often bendable?

Metal
What do we call the energy levels where electrons are arranged around the nucleus of an atom?
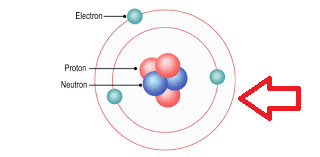
Electron shells
What do we call a vertical column on the periodic table?
Group
What do we call the type of chemical bond formed when two atoms share electrons?
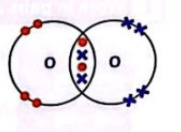
Covalent bond
What type of element is usually dull, does not conduct electricity well, and breaks easily?
Non metal
What do we call the group of elements in the last column of the periodic table that do not easily react with other elements?
Noble gas
What do we call an atom or molecule that has an electric charge because it gained or lost electrons?
Ion

Six Seven haha
What is the furthest shell away from the nucleus?
Valence shell (outer shell)
What do we call the type of chemical bond formed when one atom transfers electrons to another atom?
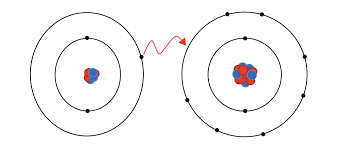
Ionic bond
What do we call a positively charged ion formed when an atom loses electrons?
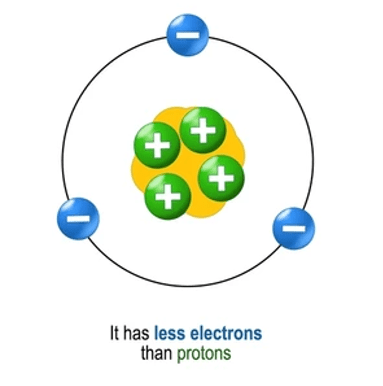
Cation
What do we call the combination of symbols and numbers that shows which elements are in a compound and how many atoms of each are present?
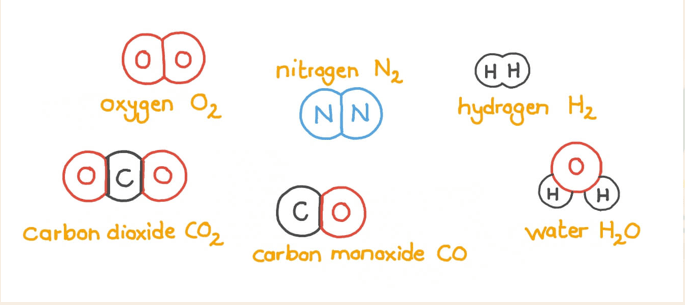
Chemical formula
What do we call a negatively charged ion formed when an atom gains electrons?
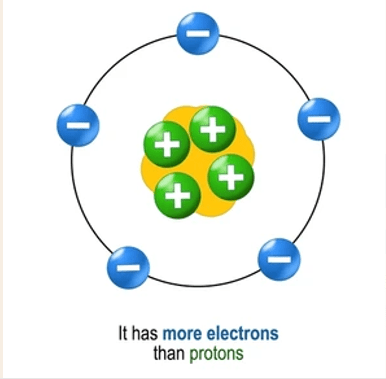
Anion
What do we call a substance made of two or more different elements that are chemically joined together?
ex. water
Compound
A property of an element that tells us how many other atoms it can combine with to form a compound
Valency
What do we call the electrons that two atoms use together when they form a covalent bond?
Shared electrons
Scientists use e______ s_______ to show the arrangement of electrons in an atom
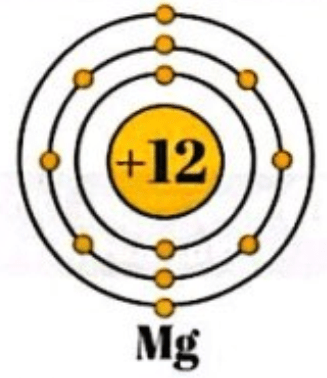
Electronic structure
What do we call a molecule made of two atoms that are chemically bonded together?
Diatomic molecule