a change in the size, shape, form, or state of matter that does not change the identity of the matter.
What is a physical change?
a change in matter in which the substance that makes up the matter change into other substances.
chemical change
Which reaction can be reversed? Physical or Chemical
What is a Physical reaction?
a positively charged particle in the nucleus of an atom.
proton
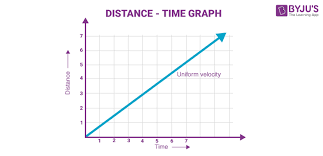
Type of motion that this graph indicates
Constant motion (speed)
The temperature at which an element or compound changes from solid to liquid.
What is the melting Point?
When heat moves from two things at different temperatures and must b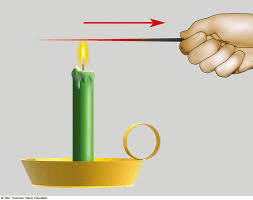 e touching
e touching
What is conduction?
A chemical reaction where heat is absorbed and the surrounding temperature falls
What is endothermic?
Number of protons, neutrons and electrons in Potassium
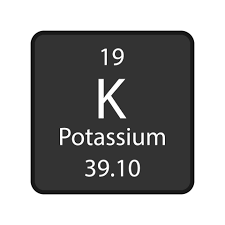
19 - protons
19 - electrons
20 - neutrons
Point where KE is highest and lowest
Kinetic Energy highest - Point X
Kinetic Energy lowest - Point W
Mass per unit volume of an object as compared to pure water.
What is Density?
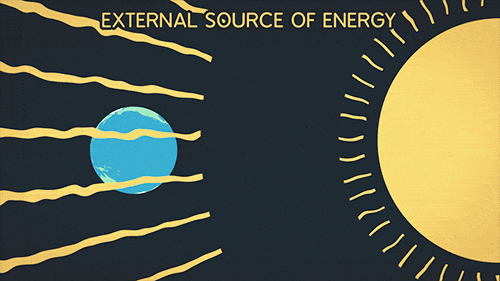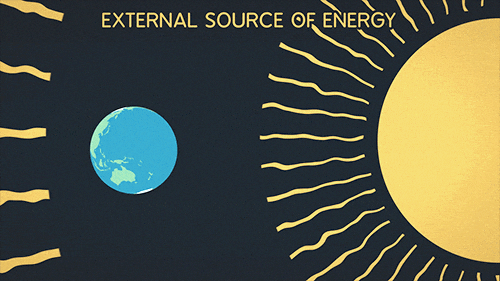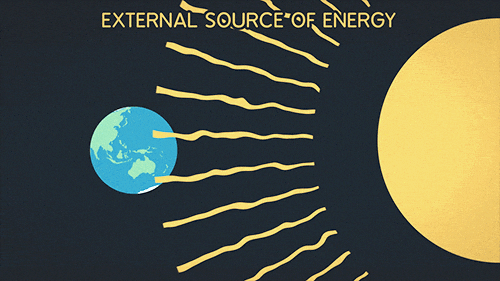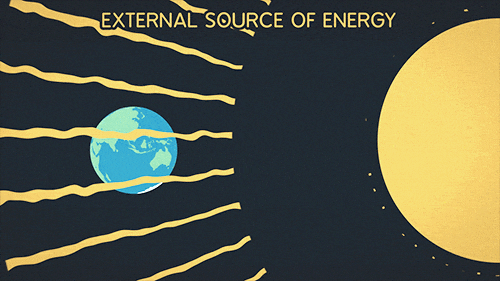 Heat transfer of energy that comes from the sun
Heat transfer of energy that comes from the sun
What is Radiation?
What is an example of a chemical change?
change in color, produces gas and heat

Identify the element from this Bohr model
Calcium
This type of substance speeds up a chemical reaction without itself being altered.
What is a catalyst?
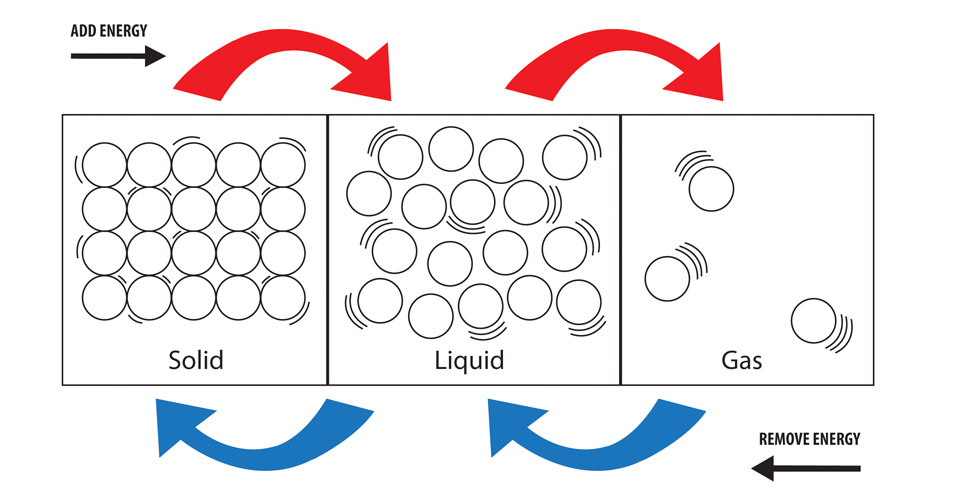 This happens to particles as energy is removed.
This happens to particles as energy is removed.
Kinetic energy decreases and particles move closer together (volume decreases and density increases)
Energy stored in the nucleus of an atom.
What is nuclear energy?
When two reactants form a single product.
What is a synthesis reaction?
Between the following elements, this one is a poor conductor of heat.
F - K - Fe - Ag
F - Fluorine?
Point with highest and lowest potential energy
PE highest at W
PE lowest at X
The density of object with a mass of 0.040kg and a volume of 20mL in g/cm3.
What is 2g/cm3.
0.040kg -> 40 g
20mL -> 20cm3
A student charges their cellphone. Shortly after the student receives a phone call. Name the energy transformations involved (4).
What is electrical current to potential chemical to electromagnetic to sound.
This is how you would correctly balance the following equation. This is the type of chemical reaction.
Al2O3 +NaCl -> AlCl3 + Na2O
What is
Al2O3 + 6NaCl > 2AlCl3 + 3Na2O?
What is a double replacement reaction?
Energy movement involving the transfer of electrons
What is ionic bonding?
Motion happening between 5:00 and 5:30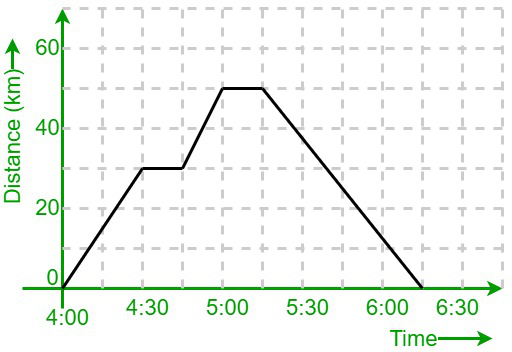
At rest