Each atom of this element has 20 protons in the nucleus
Calcium
H2O
water
a change in matter in which the substance that makes up the matter change into other substances.
chemical change
The number of protons in an atom of an element.
atomic number
a substance containing atoms of two or more different elements chemically bonded together.
compound
The temperature at which an element or compound changes from solid to liquid.
Melting Point
neither acid or base - pure water-PH=7
neutral
the ability of a substance to be hammered or rolled into sheets.
malleability
a row of the periodic table
period
How to find the number of neutrons in an element?
Subtract the atomic number from the atomic mass
mass per unit volume of an object or substance
density
NaCl
table salt
# of protons that are in an atom of Zn
30
a positively charged particle in the nucleus of an atom.
proton
a negatively charged particle that occupies the space in an atom outside the nucleus
electron
Chemical or physical property?
rust, flammability, combustibilty, acidity, basicity
chemical
new substances formed in a chemical reaction
products
made up of two or more substances but not chemically combined
mixture
CO2
carbon dioxide
Which reaction can be reversed? Physical or Chemical
Physical
Chemical or Physical Property?
malleability, ductility, solubility, density
Physical Property
a neutral particle in the nucleus of an atom
neutron
the ability of a substance to be pulled into thin wires
ductility
Number of protons, neutrons and electrons in Potassium
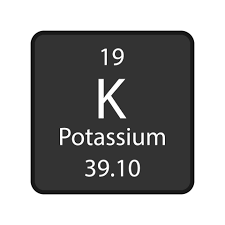
19 - protons
19 - electrons
20 - neutrons
a change in the size, shape, form, or state of matter that does not change the identity of the matter.
physical change