What is the difference between an element and a compound?
An element is just one element whereas a compound is more than one to make up one thing.
List 3 physical properties.
What is colour, ductility, malleability, boiling point, melting point, luster, hardness, etc.
What does WHMIS Stand for?
What is Workplace Hazardous Materials Information System?
How do you find the number of neutrons in a substance?
atomic mass - atomic number
What is the difference between a group and a period?
a group runs left to right and a period runs up and down.
What would you describe iced tea as?
What is a solution?
Identify a physical property of water.
Boiling point = 100 C
Melting point = 0 C
Why do you always add acid to water?
What is acid reacts strongly with water and can create a harmful reaction.
What is the difference between and electron and a valence electron?
A valence electron occurs in the outermost shell of the atom.
What element is period 3 group 17?
What is Chlorine?
What is the difference between a homogenous and heterogenous mixture?
Homogenous is a mixture where you cannot tell what is all in the mixture and heterogenous is a mixture where you can see all of its components.
What are three signs of a chemical change?
What is:
1. bubbles/gas
2. Change in colour
3. Change in smell
4. Change in temperature
5. Solid forms
Describe 3 labs rules to follow.
1. Tie hair back.
2. No food or water.
3. Wear eye protection.
4. Read through the lab before starting.
5. No running.
What is an isotope? List one thing that makes an isotope different.
What is different versions of an element with a different atomic mass and number of neutrons but has the same number of protons.
Why are the group 18 elements special?
They have a full outer shell of valence electrons so they don't like to bond with other elements.
2(SO4)?
What is 8 oxygen atoms?
Provide an example of a physical change.
Water evaporating, ice melting, etc.
What does MSDS stand for?
What is material safety data sheet?
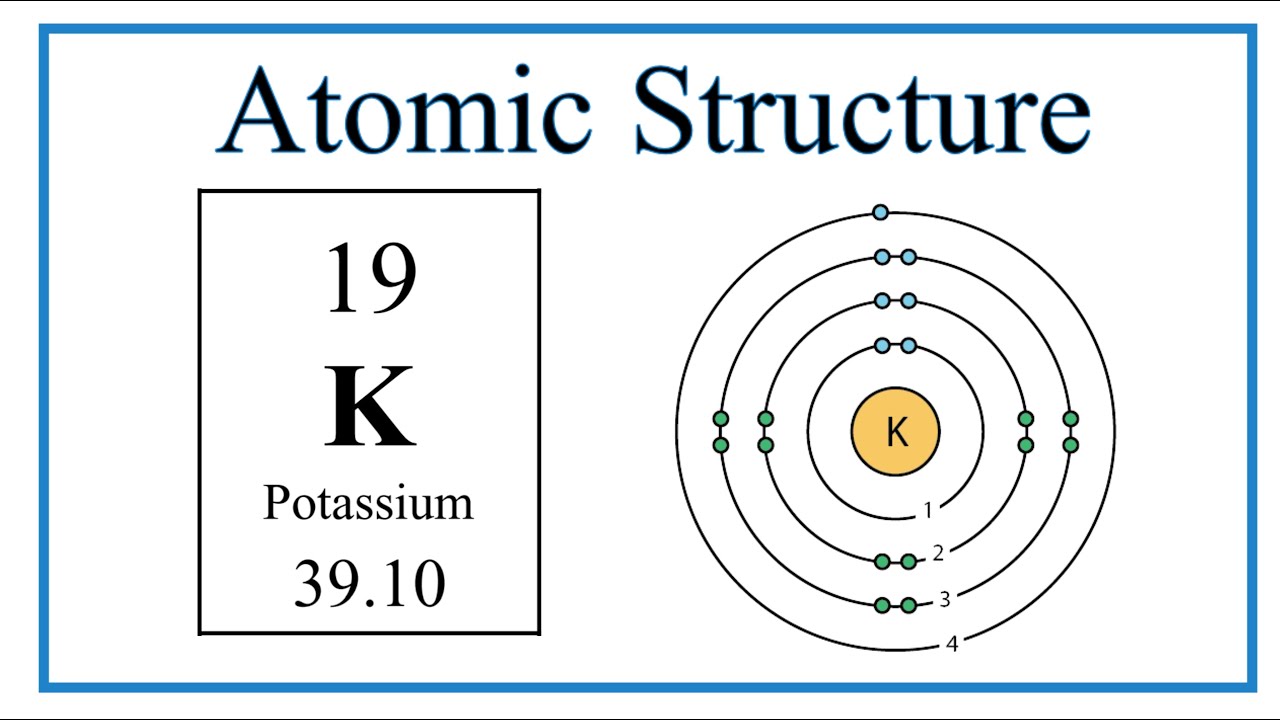
Draw the Bohr Model of Potassium.
What is a diatomic element? Tell me one of them on the periodic table.
An element that naturally occurs as a pair (oxygen, fluorine, iodine, hydrogen, nitrogen, bromine & chlorine)
Tell me how many of each atom are in the following:
3(H2SO4)
H = 6
S = 3
O = 12
Is fire burning a log a physical or chemical change? Why?
Chemical change, the log is burned and the substance changes to ash. There is a new substance formed which is the ash.
What does this image mean?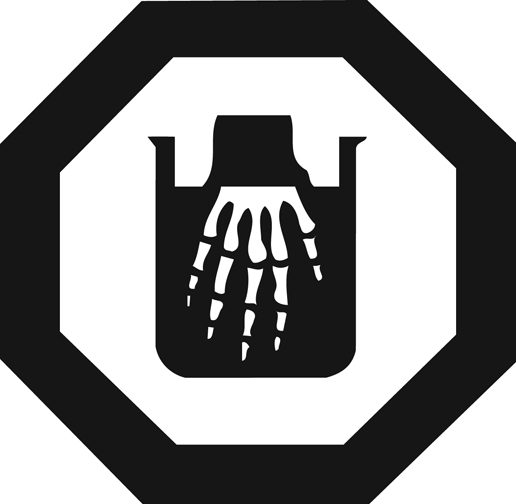
What is Danger corrosive?
What can a period and a group number tell us about our Bohr model?
Period: number of electrons shells
Group: number of valence electrons
What is a chemical property of group 1 elements?
They are very reactive.