What type of bond is formed when electrons are transferred from one atom to another?
Ionic Bond
Covalent bonds _______ (are/are not) good conductors of electricity due to _______.
are not
lack of metal in covalent bonds
What is the proper nomenclature for H20?
Dihydrogen monoxide
What does the Law of Conservation of Matter state?
Matter cannot be created or destroyed, only change in form.
Balance the equation: H₂ + O₂ → H₂O
2H₂ + O₂ → 2H₂O
Describe the main difference between ionic and covalent bonds.
Ionic bonds involve the transfer of electrons, while covalent bonds involve the sharing of electrons.
Which compound type generally has higher electrical conductivity in solution, ionic or covalent?
Ionic
Write the formula for carbon dioxide.
CO₂
What type of reaction is this? AB → A + B
Reaction Type: Decomposition reaction
Balance the equation: Na + Cl₂ → NaCl
2Na + Cl₂ → 2NaCl
How do sodium and chlorine bond? What type of bond is present?
Sodium transfers an electron to Chlorine.
Ionic
Explain why ionic compounds have high melting points.
Ionic compounds are composed of metals and nonmetals. Metals cause the compounds to have high melting points.
What is the name of NaCl?
Sodium Chloride
According to the law of conservation of matter, the mass of the reactants must be ____ to the mass of the product.
equal
Balance the equation: Fe + O₂ → Fe₂O₃
4Fe + 3O₂ → 2Fe₂O₃
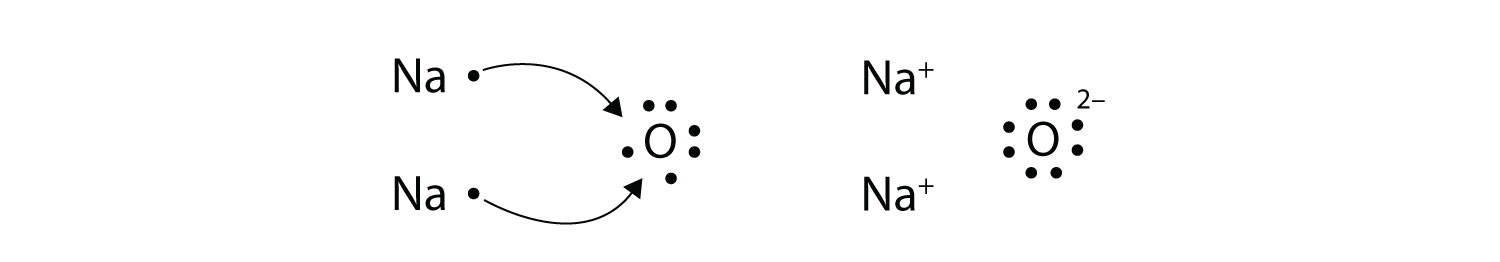
Construct a Lewis Dot Structure for sodium and oxygen.
Predict the type of bond in a compound formed between Iodine and Chlorine.
Covalent Bond
What is the name and formula for a compound of magnesium and chlorine?
Magnesium chloride
MgCl₂
What type of reaction is this? A + B → AB
Reaction Type: Synthesis reaction
Balance the equation: C₃H₈ + O₂ → CO₂ + H₂O.
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O.
Construct a Lewis Dot Structure of Hydrogen and Oxygen.

Analyze the boiling point of water (H₂O) compared to sodium chloride (NaCl).
Sodium chloride (NaCl) has a higher boiling point than water (H₂O) due to metals present in an ionic bond.
Write the formula for pentacarbon tetrachloride.
C5Cl4
What type of reaction is this? AB + CD → AD + CB
Reaction Type: Double replacement reaction
Balance the equation: KClO3→KCl+O2
2KClO3→2KCl+3O2