3 ways to increase the rate of dissolving.
What is increasing temperature, agitation (stirring), and increasing surface area.
What is the smallest unit of matter that retains the chemical properties of the element to which it belongs?
Atoms
I am the only nonmetal located in the "metal" area of the Periodic Table.
Hydrogen
Which term refers to the tendency of an object to maintain its state of motion?
Inertia
What letter represents the wavelength?
E
The amount of solute that can be dissolved in a solvent at a particular temperature.
What is "solubility"?
Which three particles make up an atom?
Protons, neutrons, and electrons
What is the number called on the Periodic Square that determines the identity of an atom?
Atomic number
Which of Newton's 3 laws explains why people in a moving car continue moving forward, even when the car stops?
Newton's 1st Law
Which term best describes the process that is occurring when light bends as it enters a new substance?
Refraction
The amount of KClO3 that is needed to create a saturated solution at 70℃?
What is 30 grams?
What do group numbers on the periodic table identify?
Number of valence electrons
What are the group of elements along the staircase on the Periodic Table?
Metalloids
According to Newton's second law, what happens when the same force is applied to two objects of different masses?
A. The object with greater mass will experience a smaller acceleration.
B. The object with greater mass will experience a greater acceleration.
A. The object with greater mass will experience a smaller acceleration.
Which is NOT a mechanical wave?
-Sound Waves
-Ocean Waves
-Seismic Waves
-Electromagnetic Waves
Electromagnetic Waves
The status of a solution with 61 grams of CaCl2 at 10 degrees Celsius.
What is unsaturated?
Darnell draws a picture of a nitrogen atom (Atomic number of 7). He draws a small circle to represent the nucleus and draws dots around the circle to represent the electrons. How many dots does he draw?
7 dots
What is the 2 step process you must do in order to find the number of neutrons in an atom?
Round the average atomic mass and subtract the protons from the mass number
A box is initially at rest on a frictionless surface. It then experiences two forces, as shown in the picture. Which best describes what will happen to the box?
A. It will move at a constant speed to the right.
B. It will move at an increasing speed to the left.
C. It will move at an increasing speed to the right.
C. It will move at an increasing speed to the right.
A student is making a model of the electromagnetic spectrum. How would the energy of the wave be described at "Label 1"? (High or Low)
Low energy
You test the pH of a solution and it reads 13 on the pH scale. This solution has a lot of ___________ ions in it.
What is hydroxide? or OH-
The illustration below, taken from the periodic table, provides information about the element calcium (Ca). How many neutrons does Calcium have?
20 Neutrons
Which elements are typically found on the right side of the Periodic Table and accept extra electrons?
Nonmetals
The table below shows the mass and velocity of four objects. Which object has the least inertia?
Object Z
The picture below shows a straw that is placed into a sports drink. Which wave behavior makes the straw look bent once it's placed in the sports drink?
Refraction
The pH of a solution was determined to have a lot of hydronium or hydrogen ions. The pH was most likely _______ than 7 on the pH scale.
What is less than?
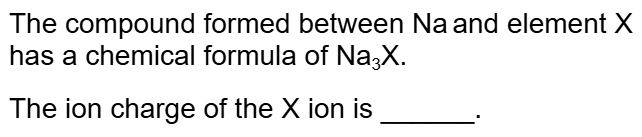
What is 3-?
This isotope has ____________ neutrons in its atom.

What is 42?
The description of motion in 3-4 in the first graph and 1s to 3s in the second graph.
What is acceleration?
In the Doppler Effect, the _________ of sound increases as the source approaches the observer.
What is pitch or frequency?





