Which scientist discovered the electrons using the Cathode-ray tube experiment?
J.J. Thomson
How many electrons maximum can exist in n = 3 shell?
18
What is 'ionization energy'?
energy needed to remove 1 electron from an atom.
What instrument can we use to measure the mass of each isotope present in an element?
Mass spectrometer
What is the name of this hydrate:
CoCl2 * 6H2O
Cobalt (II) chloride hexahydrate
Name one difference between a deuterium atom and hydrogen atom.
Different mass, deuterium has 1 neutron, different physical characteristics
Why aren't emission spectrum continuous?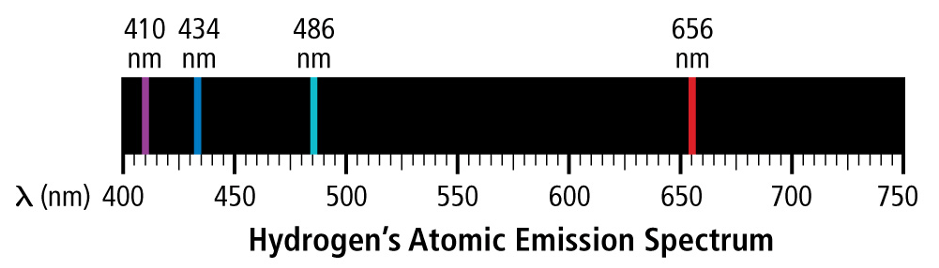
Electrons exist in quantum shell (energy level)
Inverse relationship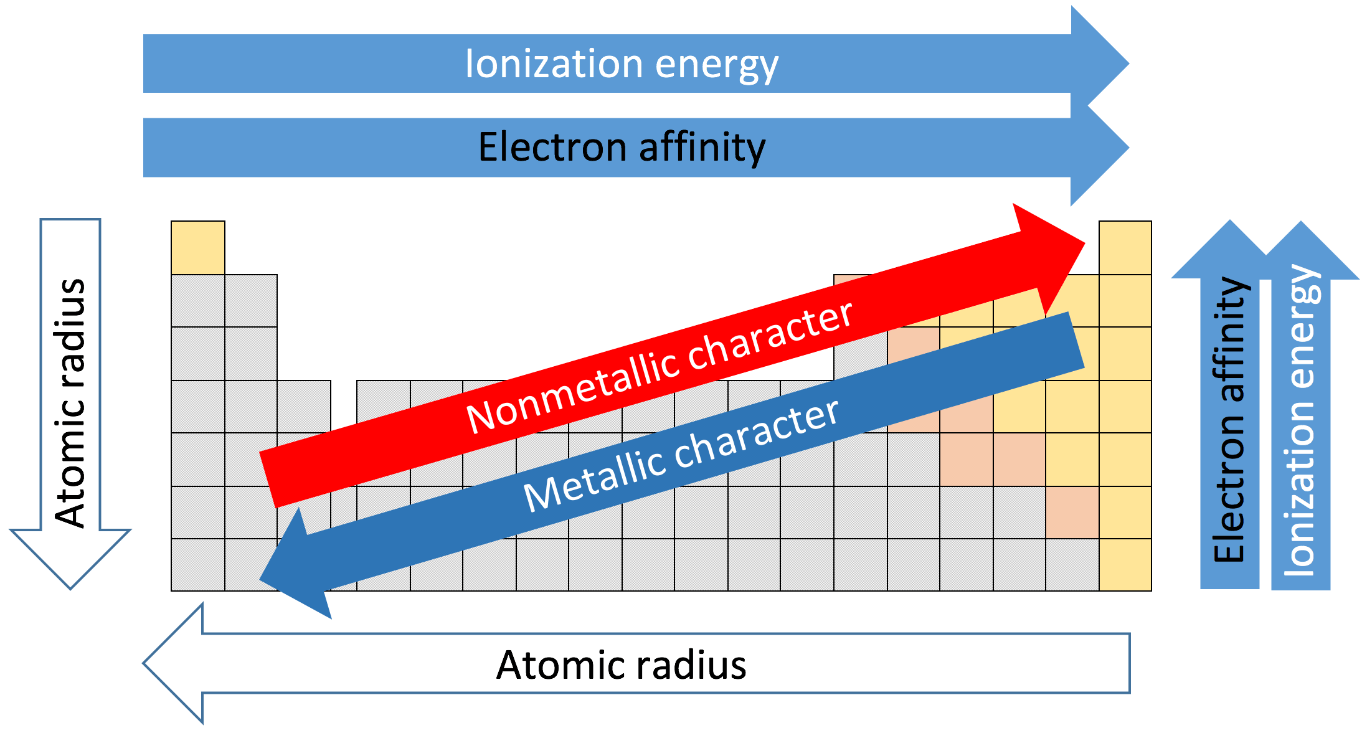
What is the volume of one mole of gas at r.t.p?
24dm3
What are the products of a combustion reaction?
CO2 + H2O
Which way would a beam of protons bend in a Cathode-ray tube experiment?
Cathode (negative plate)
How many electrons can a d sub-shell contain?
10
What factors influence the ionization energy of an atom?
atomic radius, magnitude of nuclear charge, shielding effect, electron configuration (spin-pair repulsion, stability - filled orbitals 'Mg has higher IE1 than Al because of stable e- conf)
Use the % abundance of each isotope to calculate the relative atomic mass of germanium.
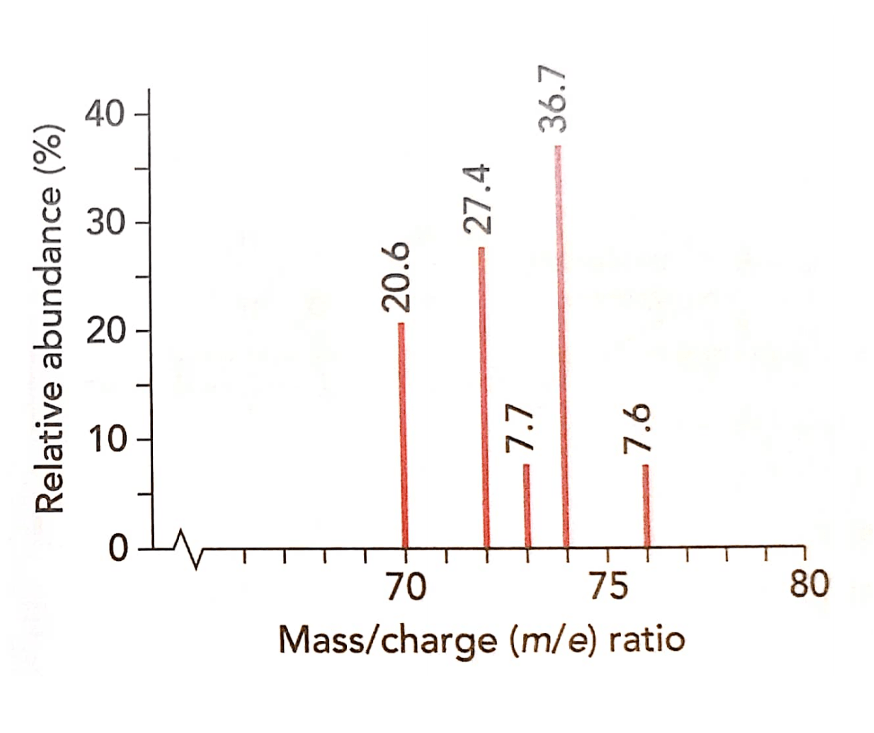
72.703
[(70 * 20.6) + (72 * 27.4) + (73 * 7.7) + (74 * 36.7) + (76 * 7.6)] / 100
What ionic compound will be produced in a neutralization reaction between sulfuric acid and ammonium hydroxide?
Ammonium sulfate
H2SO4 + NH4OH --> H2O + (NH4)2SO4
Explain how nucleon number can be calculated.
protons + neutrons
What is the electron configuration of sulfur?
1s2 2s2 2p6 3s2 3p4
How would you write an equation of IE3 for lithium atom?
Li2+(g) --> Li3+(g) + e-
What is the empirical formula of a compound containing 85.7% carbon & 14.3% hydrogen?
(C = 12, H = 1)
CH2
C: 85.7/12 = 7.142/7.142 = 1
H: 14.3/1 = 14.3/7.142 = 2
Calculate the relative atomic mass of the following sample of lithium: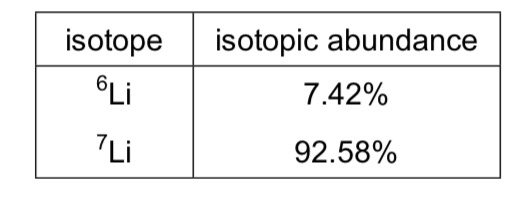
6.93
[(6 * 7.42) + (7 * 92.58)] / 100
How many electrons are present in this ion?
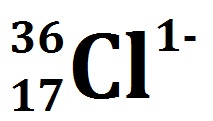
18
What is the noble gas configuration of copper?
[Ar] 3d10 4s1
* swap the 3d & 4s spot
* better if the d-subshell is filled or half filled
The successive IE of element X are shown in the table below. Which group do you think element X belong to in the periodic table?
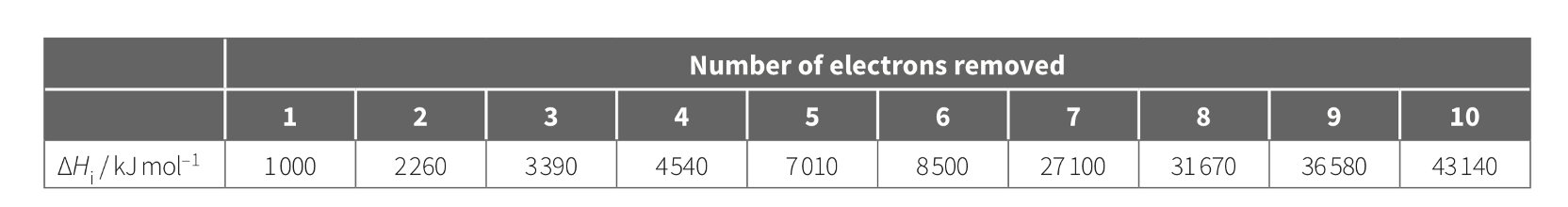
Group 6
(there're 6 valence electrons - lowest 6 IE and jump at 6-7)
What would be the ration of the M+ and [M+2] peaks of an organic compound containing one chlorine atom?
3:1
Cl-35 (M+): 75% abundant
Cl-37 (M+2): 25% abundant
What would be the ration of the M+ and [M+2] peaks of an organic compound containing one chlorine atom?
3:1
Cl-35 (M+): 75% abundant
Cl-37 (M+2): 25% abundant