A scientific ________ predicts what will happen and is often associated with a mathematical formula
Law
When should you end a measurement?
When you write down everything you know and estimate one more digit.
How many sig figs in a measurement of 10.050 seconds?
5
SI unit for mass
kilogram
How many meters are in a kilometer?
1000
The term used for when multiple measurements are close to the same value.
Precision
How many mL of liquid are in the graduated cylinder?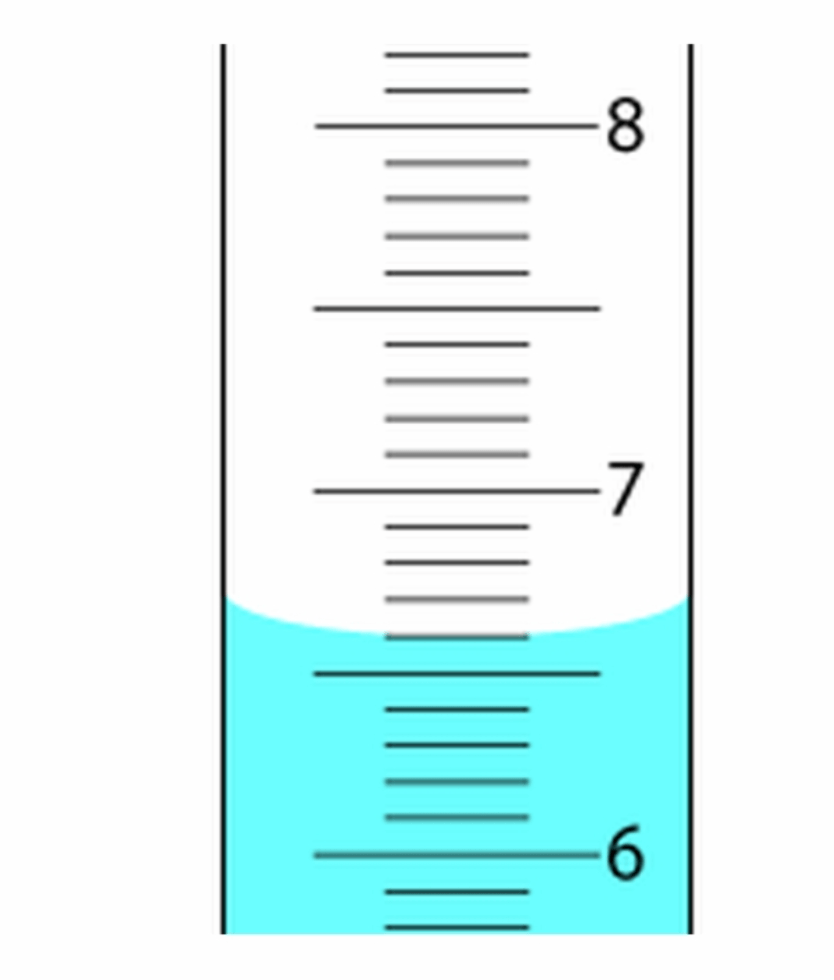
6.60mL
Write 0.00240 sec in scientific notation.
2.40 x 10-3 sec
SI unit for temperature
Kelvin
How many atoms of carbon in one mole
6.02 x 1023
The variable that is measured
Dependent
What is the length of the wood in mm?
45.8mm to 46.0mm
Unlimited
What does volume measure?
amount of space that something takes up
How many grams are in 2.5 moles of fluorine?
48 grams
Part of the experiment that is not manipulated so it may be used for comparison
Control group
Which ruler would give you more precision in your measurement and how do you know?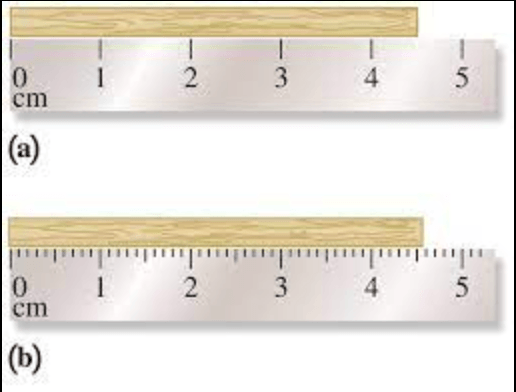
Ruler B - There would be a smaller difference in the measurements because you could measure to an additional decimal place.
A student measures a mysterious block and finds it has a mass of 16.84g and a volume of .56cm3. What is the density of the block?
3.0 x 101g/cm3
What is the difference between mass & weight?
Mass = amount of material in a substance
Weight = pull of gravity on that substance.
How many atoms are in 72.8g of silicon?
1.56 x 1024 atoms
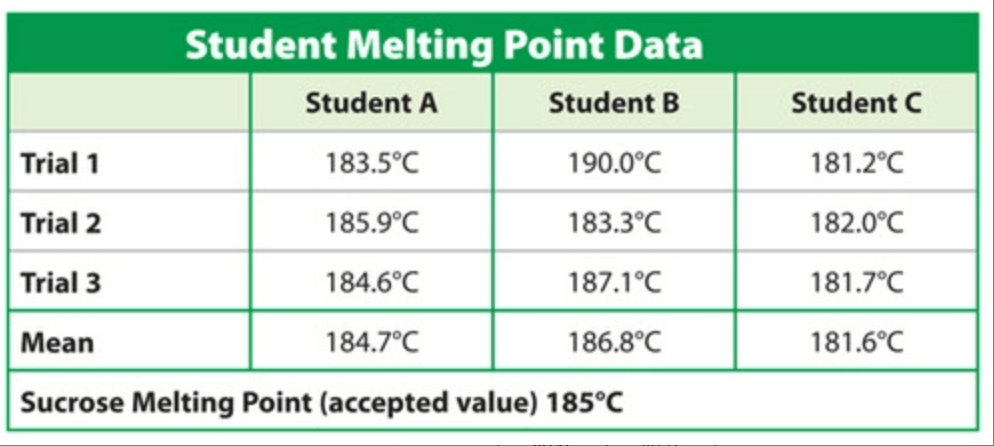
Find the percent error for the student that is the most accurate.
0.162%
A student does a lab and calculates that his experiment has produced 3.82g of lithium. It was supposed to produce 1 mole. What is his percent error with the amount of atoms?
45.0% error
A student has 1 mole sample of iron filings. If they use 4.6 x 1022 atoms for an experiment. What percentage of atoms did they use?
7.6% are used.
A block has a length of 0.25 cm, a width of 1.56 cm and a height of 1.10 cm. The mass is 3.745 g. Determine the density of the block.
8.7 g/cm3
A piece of magnesium takes up 0.560cm3 and has a density of 1.739g/cm3. How many atoms are in the sample?
2.41 x 1022 atoms