Which of the following are NOT a sign of a chemical reaction? (only 1 option)
* Change in temperature
* Change in smell/odor
* New gas produced
* Light emitted
* Phase change (solid<->liquid<->gas)
* A precipitate is formed
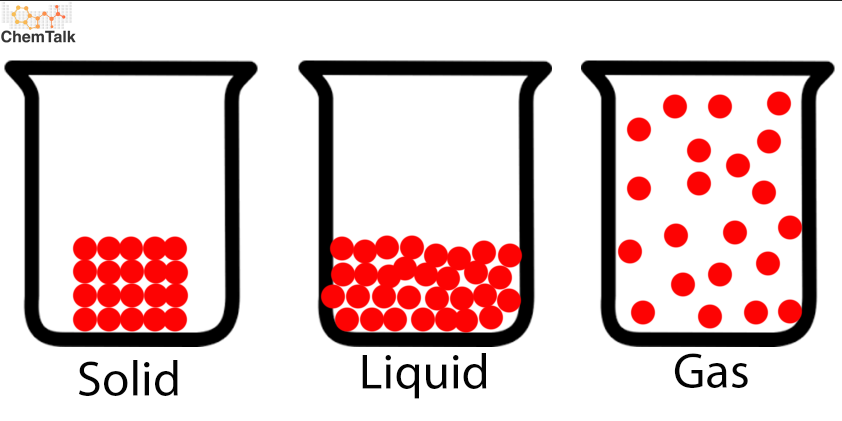
Phase changes are physical changes! Ice is water and steam is also just water!
Because almost all the alpha particles traveled through gold foil in Rutherford's experiment, we learned that atoms are mostly just...
empty space.

True or false? The speed of light is constant in this universe.
Very very true.

Cations have a ______________ charge.
Anions have a ______________ charge.
Cations have a positive charge. These are metals.
Anions have a negative charge. These are nonmetals.
What is the chemical name for Na2O?
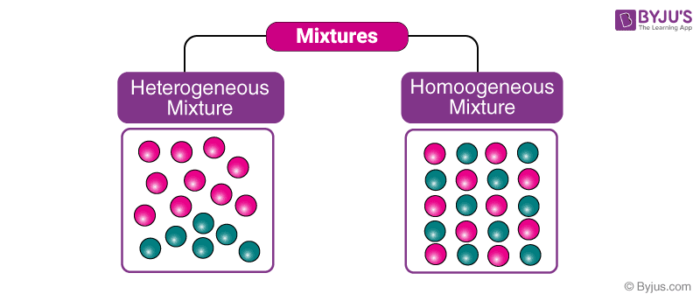
Draw a particle diagram of any heterogenous mixture you can think of.
Which of the following elements are halogens?
H, F, Ne, Ca
Fluorine

As the wavelength of light increases...
the frequency will ____________
the energy will ____________
As the wavelength of light increases...
the frequency will DECREASE
the energy will DECREASE
An ionic bond is formed between a __________ and __________.
A covalent bond is formed between a __________ and __________.
1. metal and nonmetal
2. nonmetal and nonmetal (metalloid counts here too)
What is the chemical formula for Lithium sulfide?
Li2S
How many hydrogen atoms are there in...
5 (NH4)2O
40
List all of the diatomic molecules that always exist naturally as gases.
HONClBrIF
Correctly assign the energy transitions X, Y, and Z with waves 1, 2, and 3.
X = 1
Y = 2
Z = 3
Which element is more reactive and why? Write at least two reasons on your board. Lithium vs. Sodium.
Sodium will be the more reactive metal due to having:
* Larger radius
* Weaker nuclear charge
* Lower ionization energy
* Weaker electronegativity
All these answers count.
What is the chemical name for Ba3S2?
Provide an example of a substance that is a molecule but not a compound.
Anything diatomic!
Iron has 5 naturally occurring isotopes. Which one is most commonly seen in the universe? Cite your evidence on your board.
Fe-54, Fe-55, Fe-56, Fe-57, Fe-58
Iron-58 is the most abundant because the average atomic mass of Iron is 57.933 which is really close to the mass number 58. The closer the mass number is to the the average atomic mass, the more abundant that isotope is.

Write the electron configuration for bromine.
DAILY DOUBLE! Get this correct and get double points.
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵
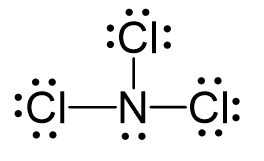
Draw and determine the molecular geometry (shape) of NCl3. Then state whether it is polar or nonpolar.
Trigonal pyramidal, polar.
What is the chemical formula for tetraphosphorus decahydride?
P4O10
You must draw 3 particle diagrams:
1. Pure element
2. A mixture of two elements
2. Pure compound
gonna have to draw this on the board

Mass number = 18
Atomic number = 8
Charge = 1-
Write the electron configuration for Calcium and then draw its orbital diagram.

Draw and determine the molecular geometry (shape) of KrF2. Then state whether it is polar or nonpolar.
Linear, nonpolar.

What is the chemical name of the compound Pb(CO3)2?
Lead (IV) carbonate
a type II cation and type IV anion!