What is the purpose of radioactive decay?
For unstable isotopes to become stable by emitting a kind of particle.
How do you write a ionic compound in general? What do you write first?
Cation first then Anion
Draw the stick diagram for N2
It should be a triple bond with 2 electrons on the outside for each N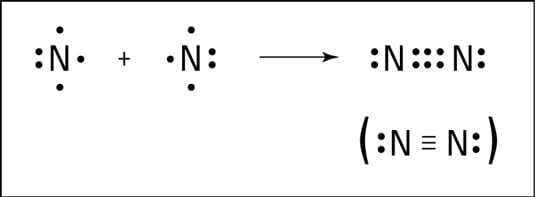
What is the difference between gas and plasma?
Identify the three subatomic particles.
Proton, Neutron, Electron
Write Germanium-70 in nuclear notation.
Write Sodium-24 in nuclear notation
7032Ge
2411Na
Why are cations and anions bonding with eachother in an ionic compound?
Cations and Anions have charges which makes them unstable. Opposite charges attract each other, therefore they cancel out to create a neutral charge.
If we have 3 domains, what is the bond angle?
120 degrees
What is the law of conservation?
Matter can never be created nor destroyed
2 electrons only!
Deuterium (or hydrogen-2), Helium-3, Helium-4
What is the name of LiNO3
Lithium Nitrate
Any halogens needs ______ additional electron(s) to complete its octet
Halogens only have 7 valence electrons. Therefore they only need 1 to make 8.
Describe what phases is used in sublimation.
Solid turning into a gas
What happens when we apply extreme heat on a metal cation?
It releases energy in the form of a photon. (colored fire lab)
Write out the equation of Iridium-191 if it were to go through beta decay.
19177Ir => 0-1e + 19178Pt
What is the formula of Sodium Carbonate?
Na2CO3
What type of diagram uses a single dot to represent an electron?
What kind of electrons are represented in this diagram?
Lewis dot diagram.
Valence electrons
Name three metalloids.
Si, Te, B, Sb, Ge, As, At, etc.
Silicon, Tellurium, Boron, Antimony, Germanium, Aresnic, Astatine.
What is the full electron configuration of Aluminum?
1s22s22p63s23p1
In gamma decay, the electrons are getting excited and release photon energy.
When do we write the roman numeral in the name of an ionic compound? What does the roman numeral represents?
We only write the roman numeral when the metal charge is variable. The roman numeral represents the charge of an ion in the compound.
What is the name, shape, and bond angle for Cl2
Which one has higher ionization energy? Hydrogen vs Cesium
Cesium has more ionization energy.
Ionization energy trend: increase from bottom to top, increases from left to right.
What is the shorthand and full electron configuration of Calcium?
Shorthand: [Ar]4s2
Fullhand: 1s22s22p63s23p64s2