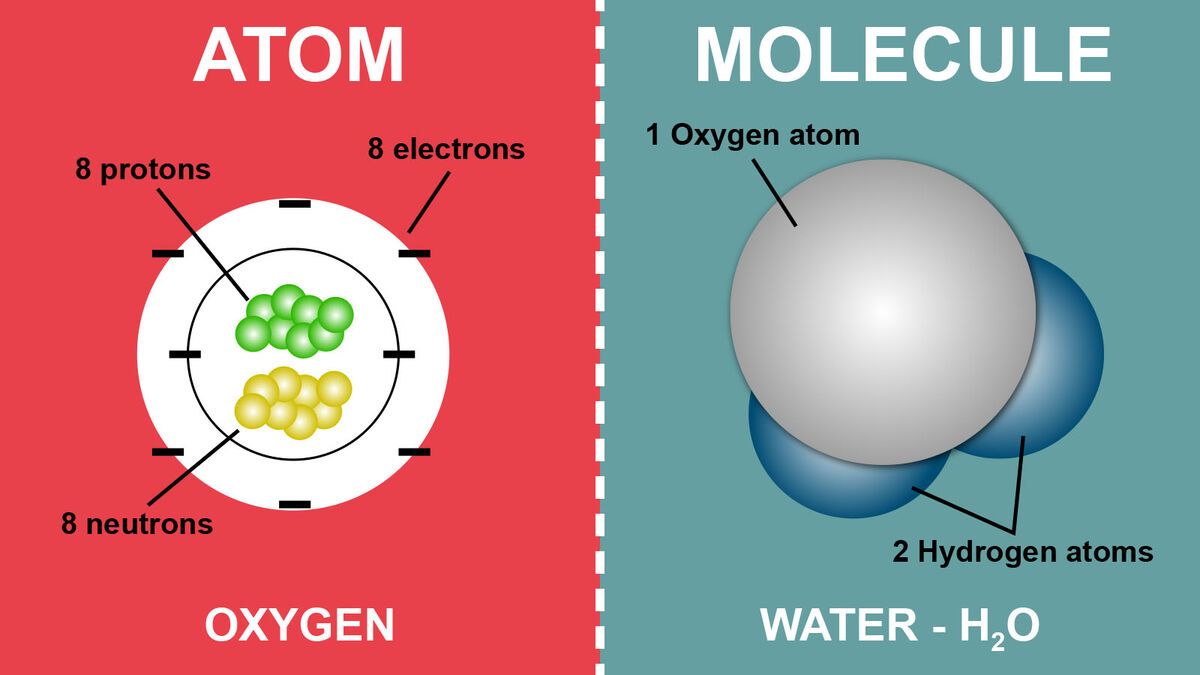
What family do Bromine (Br) and Chlorine (Cl) belong to?
Halogens

How many electrons would this molecule have?
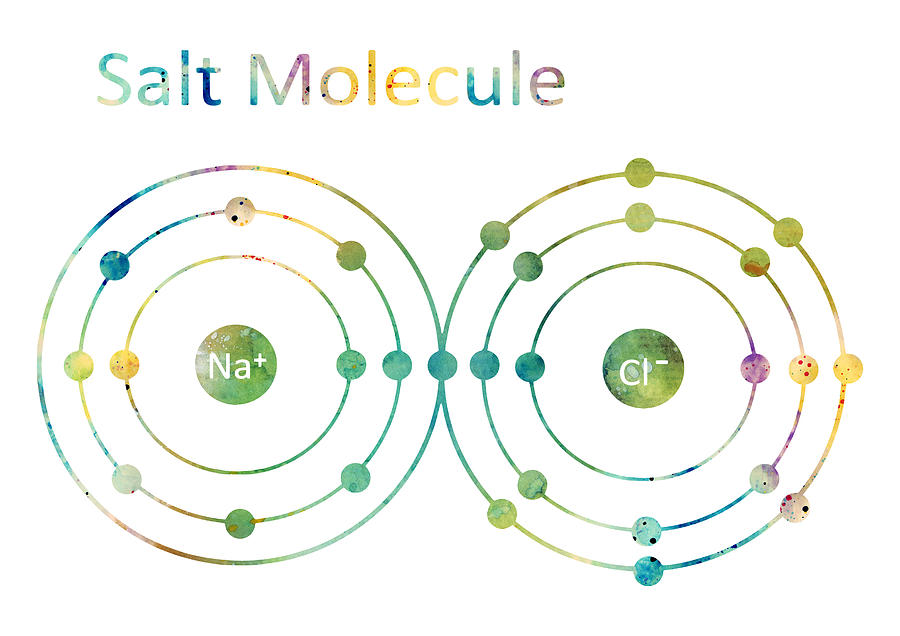
11 + 17 = 28
What is it called when water molecules gain energy and turn from a liquid to a gas?
Evaporation

What is the difference between an independent variable and a dependent variable?
Independent = You change in an experiment
Dependent = What you measure in an experiment
What is the best graph to use when dealing with percentages (100%)?
Circle Graph
What vocab word does this definition belong to:
Process by which one or more substances are altered to form new substances.
Chemical Change

How many valence electrons does Bismuth (Bi) have?
Group 15 = 5 valence electrons
What would the chemical formula be for the following molecule?
![]()
H2SO4
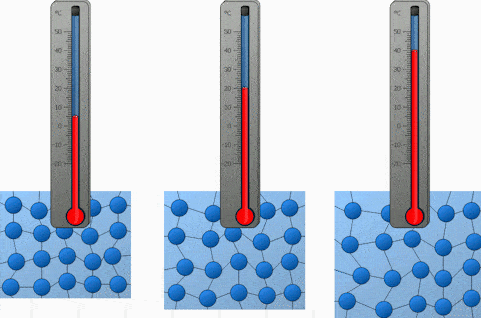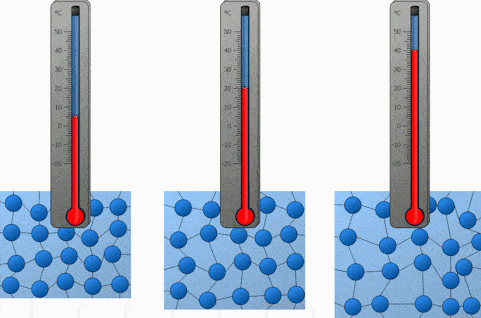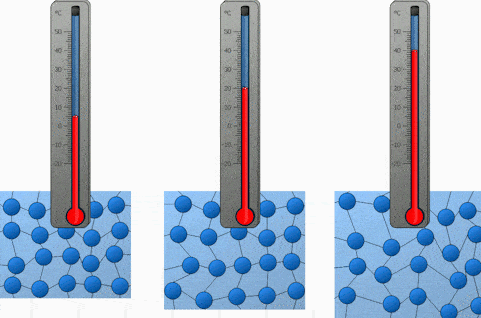
What happens to molecules when a substances is heated?
When it is cooled?
Heated = Gains Energy and Speeds Up
Cooled = Loses Energy and Slows Down
Which type of bond shares electrons between 2 non-metals?
Transferring electrons between a metal and a non-metal?
Covalent = Sharing / Non-Metals
Ionic = Transferring / Metal & Non-Metal
Carlos wanted to see if his turtle preferred lettuce to carrots. He used the same turtle and served the food at the same temperature. Carlos only conducted 1 trial for each type of food.
How can you make this experiment better?
Repeated experimentation (MORE TRIALS!!!) always makes an experiment better.
What vocab word does this definition belong to:
A substance that cannot be subdivided using chemical means; identified by the number of protons in its atoms.
Element
:max_bytes(150000):strip_icc()/GettyImages-604346724-589a56263df78caebc80b0c5.jpg)
Which Nobel Gas has the largest mass (more protons, neutrons and electrons)?
Older Table = Radon (Rn)
Newer Table = Oganesson (Og)

Write down a chemical formula for a compound?
NaCl
H2O
H2SO4
C6H12O6
And Many More!!!
What types of elements and how many of each do you need to make a molecule of water?
H2O
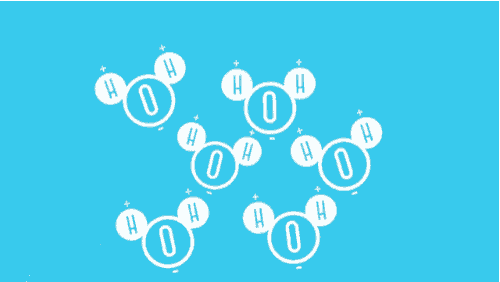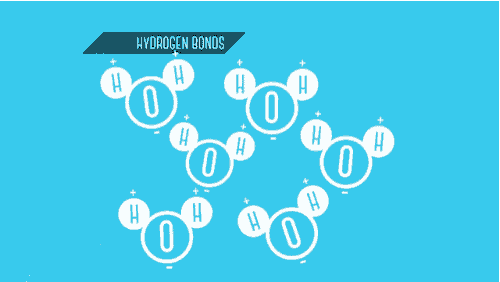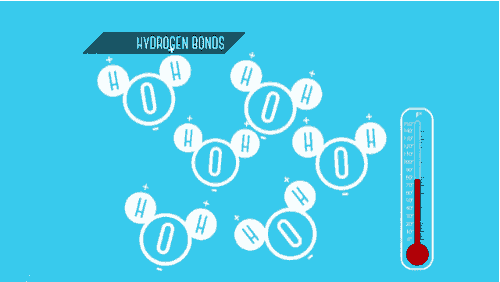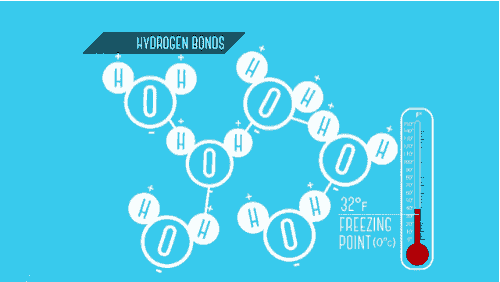
Write down a situation of a physical change?
Boiling Water
Shredding Paper
Crushing Ice
What law states that mass is neither created or destroyed during a chemical reaction?
The Law of Conservation of Mass
What vocab word does this definition belong to:
Starting material for a chemical reaction.
Reactant

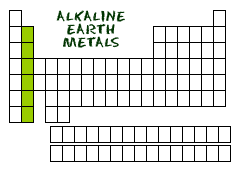
Name 2 elements that would have similar chemical and physical properties to Calcium (Ca)?
Beryllium (Be)
Magnesium (Mg)
Strontium (Sr)
Barium (Ba)
Radium (Ra)
How many protons, neutrons and electrons does Aluminum have?

Protons and Electrons = 13
Neutrons = 27 - 13 = 14
How many of each atom are in the following chemical formula? Make a list
3(NH4)3PO4
N = 3 x 1 x 3 = 9
H = 3 x 4 x 3 = 36
P = 1 x 3 = 3
O = 4 x 3 = 12
What happens to the atoms during a chemical reaction?
The atoms are rearranged to form new molecules
Write down a situation of a chemical change?
Rusting Iron
Burning Coal
Exploding Substance
What vocab word does this definition belong to:
Outer electrons most likely to participate in bond formation or a chemical reaction.
Valence Electrons
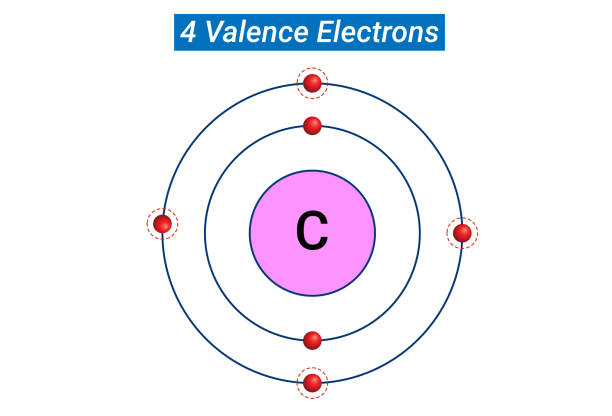
Where are the reactive metal families (Alkali and Alkaline Earth Metals) located on the Periodic Table?
Metals are to the left of the bold, zig-zag line
Reactive metals are on the far left of the line (groups 1 and 2)

Is the following chemical equation balanced or unbalanced?
CH4 + 2O2 --> CO2 + H2O
Unbalanced
CH4 + 2O2 --> CO2 + H2O
C = 1 C = 1
H = 4 H = 2
O = 4 O = 3
CH4 + 2O2 --> CO2 + 2H2O
Explain the motion of the molecules during condensation?
The gas molecules lose energy and slow down to form a liquid
What are 3 signs of a chemical reaction?
1. Color Change
2. Change in Temperature
3. Production of an Odor (Smell)
4. Formation of a Gas (Bubbles)
5. Formation of a Solid (Precipitate)
Explain what would happen to the mass (increase, decrease or stay the same) after this chemical reaction is complete?
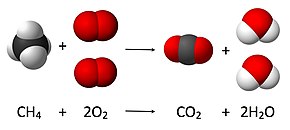
Mass is always conserved or stays the same
Law of Conservation of Mass!!!
What vocab word does this definition belong to:
Chemical species formed by two or more atoms that share chemical bonds such that they form one unit.
Molecule