What is the molar mass of NaOH?
39.997 g/mol
Which of the following is acidic?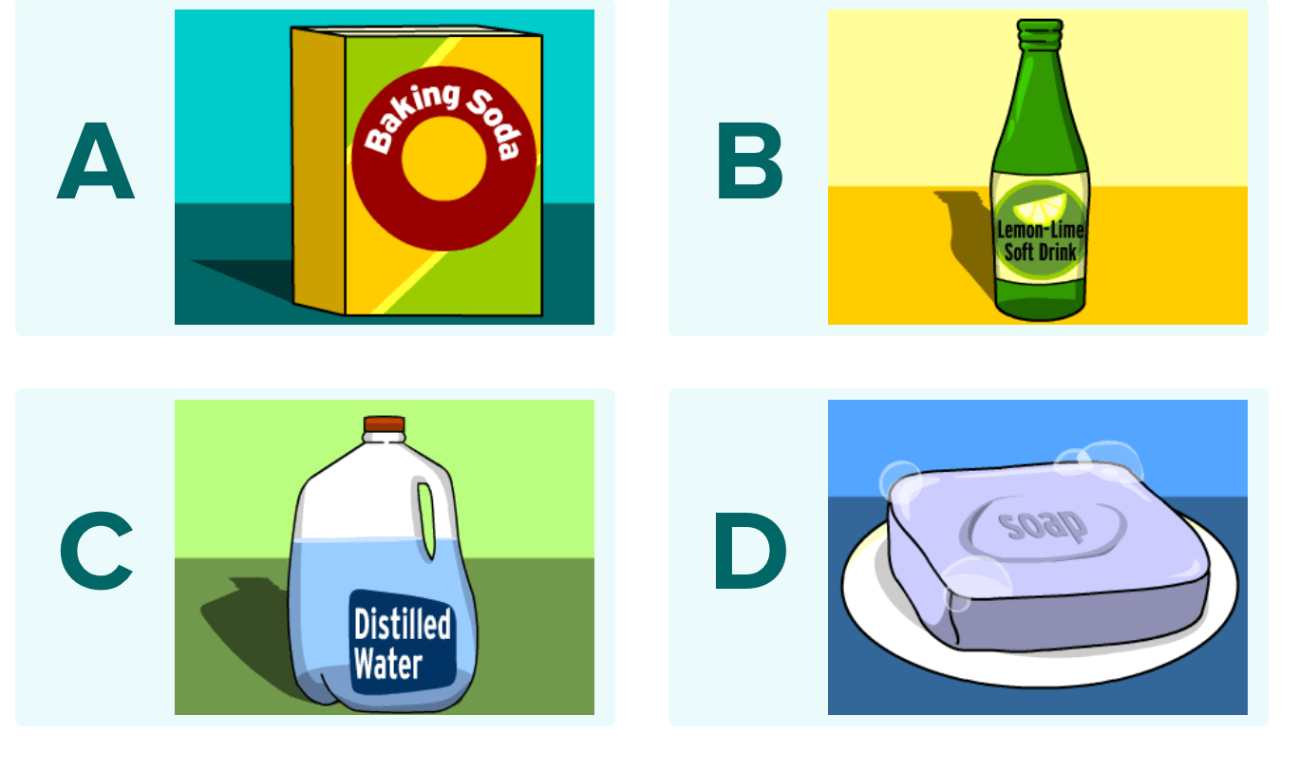
B
A bond formed when atoms share electrons unequally
polar covalent
Is completely used up in a chemical reaction
Limiting reactant
Balance the following:
H2O2 --> H2O + O2
2 H2O2 --> 2 H2O + O2
17 grams of NaCl converted to moles
(molar mass NaCl = 58.44 g/mol)
0.29 moles
What substance would do a good job of cleaning pots and pans?
A. substance with a pH of 7
B. substance with a pH of 2
C. substance with a pH of 4
D. substance with a pH of 8
D
lone pairs/unshared
In a reaction, 6.79 moles of O₂ react with H₂. How many moles of H₂O are formed?
2H₂ + O₂ → 2H₂O
What type of chemical reaction is the following?
Ag + Cu(NO3)2 --> Cu + Ag2NO3
Single replacement
The mass of 0.5 moles of NaCl.
29.25 grams
This color indicates a base on litmus paper.
blue
Name the following chemical formula:
Tetraphosphorus hexasulfide
P4S6
Calculate the number of moles, starting from 200 grams:
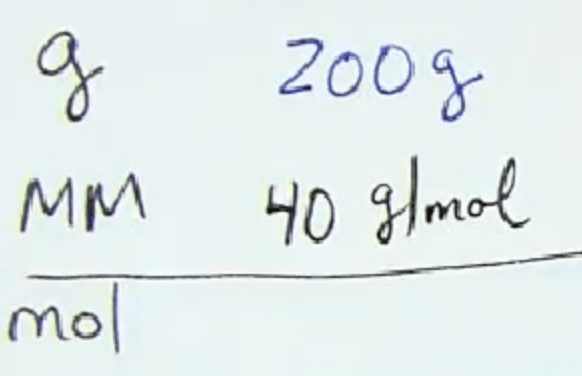
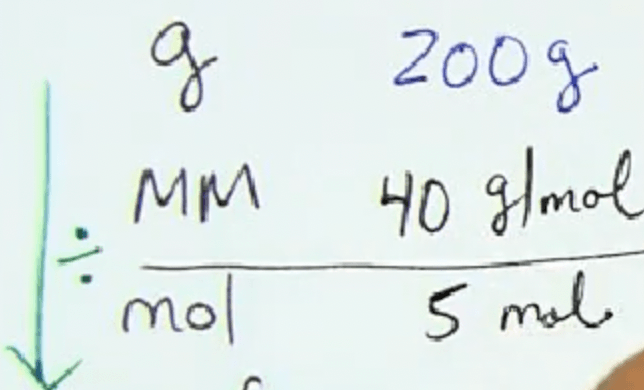
Balance the equation: ___Al + ___O₂ → ___Al₂O₃
4Al + 3O₂ → 2Al₂O₃?
You have 1.2 × 10²⁴ molecules of O₂. Convert this to moles.
2 moles
Acid or Base?
1) H3PO4
2) Ca(OH)2
1) Acid
2) Base
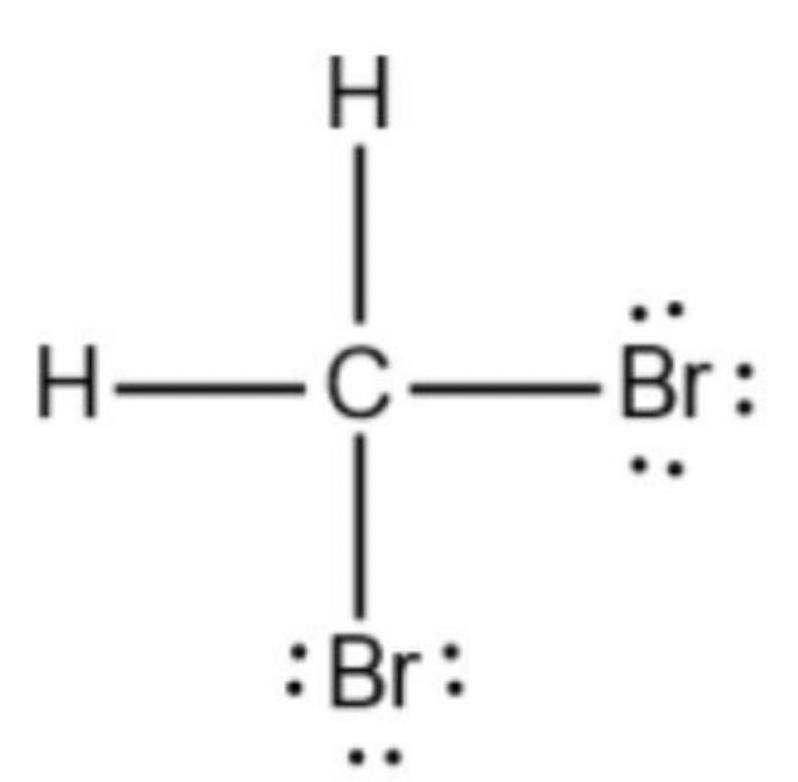
Draw Lewis Structure for CH2Br2
If in a lab a reaction yields 32 grams, but 47 grams was expected, what is the percentage yield of the reaction?
68%
Identify the reaction type and balance:
___KClO₃ → ___KCl + ___O₂
Decomposition reaction
2KClO₃ → 2KCl + 3O₂