Classify the type of bond in the compound as ionic bond or covalent bond: MgCl2
ionic bond
There is a metal in the compound
Write the chemical formula for the binary ionic compound: Sodium phosphide
Na3P
Classify the type of reaction: Sr3N2 --> Sr + N2
Decomposition
1 reactant --> 2 (or more) products
Balance the equation: __ P4 + __ Br2 --> __ PBr3
1 P4 + 6 Br2 --> 4 PBr3
Which of the two solutions is MORE concentrated?
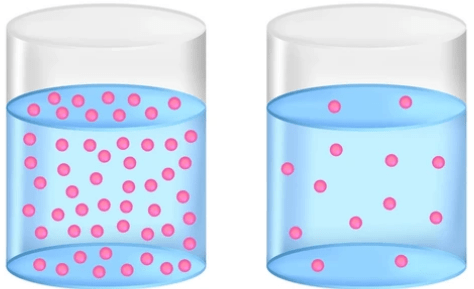
The left one
More particles = more concentrated
How many moles AlCl3 are produced from 0.31 mol of Al?
2 Al + 6 HCl --> 2 AlCl3 + 3 H2
0.31 mol AlCl3
Classify the type of bond in the compound as ionic bond or covalent bond: SO2
covalent bond
There are only nonmetals in the compound
Name the ternary ionic compound: MgCO3
magnesium carbonate
Classify the type of reaction: P4 + O2 --> P2O3
Synthesis/combination
2 reactants --> 1 product
Balance the equation: __ Fe3O4 + __ CO --> __ Fe + __ CO2
1 Fe3O4 + 4 CO --> 3 Fe + 4 CO2
The pH of a solution is measured to be 4.2. Is this solution acidic, basic, or neutral?
Acidic
How many moles of AlCl3 are produced from 0.79 mol of HCl?
3 HCl + 1 Al(OH)3 --> 1 AlCl3 + 3 H2O
0.26 mol AlCl3
Classify the compound as BIC, TIC, or BMC: Li2SO4
TIC
3 or more elements in the compound
Name the binary molecular compound: P2O7
Diphosphorus heptoxide
Classify the type of reaction: NaOH + H3PO4 --> H2O + Na3PO4
Double replacement
compound + compound --> compound + compound
Balance the equation: __ C6H12 + __ O2 --> __ CO2 + __ H2O
1 C6H12 + 9 O2 --> 6 CO2 + 6 H2O
True or False: Acids start with a H (hydrogen) and Bases end with a OH (hydroxide).
TRUE
How many grams of AlCl3 are produced from 0.79 mol of HCl?
3 HCl + 1 Al(OH)3 --> 1 AlCl3 + 3 H2O
35.11 g AlCl3
Classify the compound as BIC, TIC, or BMC: Na2S
BIC
2 elements in the compound, one is a metal
Name the compound: Li3PO4
Lithium phosphate
Li + (PO4)
Classify the type of reaction: Al + HCl --> AlCl3 + H2
Single replacement
element + compound --> element + compound
Balance the equation: sodium reacts with chlorine to produce sodium chloride.
2 Na + 1 Cl2 --> 2 NaCl
True or False: Acids taste bitter, feel slippery, and have a pH greater than 7.
FALSE. These are properties of BASES.
How many moles of AlCl3 are produced from 0.79 g of HCl?
3 HCl + 1 Al(OH)3 --> 1 AlCl3 + 3 H2O
0.0072 mol AlCl3
Classify the compound as BIC, TIC, or BMC: N2O5
BMC
2 elements in the compound, both are nonmetals
Write the chemical formula for: Ammonium carbonate
(NH4)2CO3
Classify the type of reaction: C6H12O6 + O2 --> CO2 + H2O
Combustion
something + O2 --> CO2 + H2O
Balance the equation: Dicarbon tetrahydride reacts with oxygen gas to produce carbon dioxide and water.
1 C2H4 + 3 O2 --> 2 CO2 + 2 H2O
What are the products of the neutralization reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH)?
NaCl (the salt) and H2O (water)
How many grams of AlCl3 are produced from 0.79 g of HCl?
3 HCl + 1 Al(OH)3 --> 1 AlCl3 + 3 H2O
0.96 g AlCl3