magnesium
Mg
Decide if the following is an example of inorganic chemistry, organic chemistry, analytical chemistry, biochemistry or theoretical chemistry.
Using mass spectrometry to measure charged particles to determine the composition of a substance.
analytical chemistry
What is the atomic number of silver?
47
a chocolate chip cookie is an example of a _____
mixture
Filtration
Find the state of matter of oxygen in the following chemical equation:
2 HgO(s) --> 2 Hg(l) + O2(g)
Is the following a physical or chemical property:
nitric acid reacts violently with copper
Chemical Property
All measurements taken with this rules would end in which place value and unit?

What is 100ths place and centimeters?
X.XX cm
120 g in kg
What is 0.12 kg?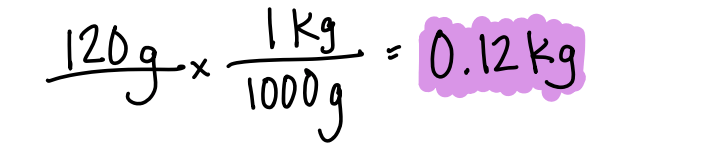
The number of sig figs in 0.02050 m
What is 4?
The volume of a 73.9 g sample of tin with a density of 7.21 g/cm3.
What is 10.2 cm3?
The molar mass of (NH4)2CO3
Is the following a physical or chemical change:
A marshmallow roasts over a campfire
Chemical Change
potassium
K
A student wants to test which kind of food makes hissing cockroaches grow the fastest. They obtain 3 roaches that are the same size, age and the same species. They put the roaches in 3 identical cages in the same room. They give them the same amount of water. They provide the first roach with apples, the second roach with dog food and the third roach with crackers. Then they measure the roaches each day to see how long they are to determine the best diet for cockroach growth.
The roach cages given is the _________________ variable.
control variable
N, P, As, Sb, Bi, or Mc
Does the following represent an element, compound, or mixture
NaOH + HCl
mixture
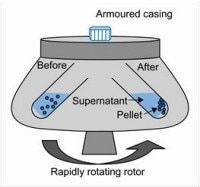
Centrifuging
Find the reactant(s) in the following chemical equation:
2 HgO(s) --> 2 Hg(l) + O2(g)
HgO
Is the following a physical or chemical change:
Na(l) → Na(s)
Physical Change
The place value and unit which this graduated cylinder reports volumes
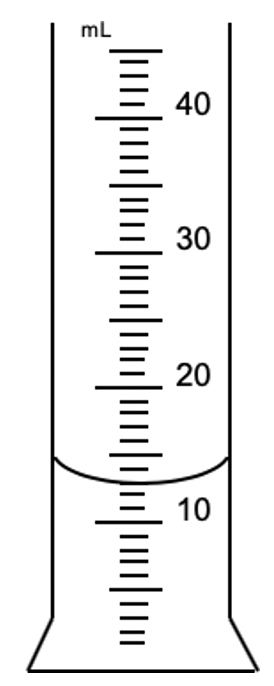
What is the 10ths place and milliliters?
13.0 mL
3.28 km in cm
What is 3.28 105 cm?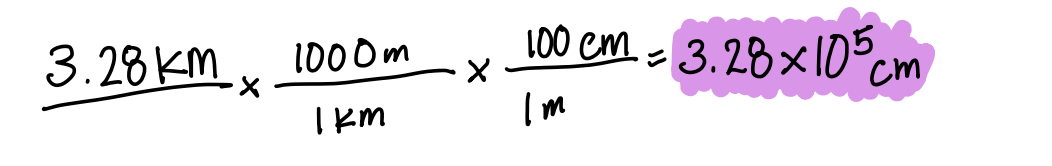
5.81 cm - 0.983 cm = ??
What is 4.83 cm?
The density of chromium is 7.19 g/cm3. The volume of a 0.31 kg sample of Cr.
What is 43 cm3?
How many atoms are in 0.750 moles of zinc?
Determine if the following elements are metals or nonmetals:
zinc (Zn), iodine (I2), strontium (Sr), radon (Rn)
zinc: metal
iodine: nonmetal
strontium: metal
radon: nonmetal
phosphorus
P4
Label the following statement as observation, hypothesis, experiment or scientific theory:
Many bugs are seen dead at the bottom of the lamp shade.
observation
What is the atomic mass of barium?
137.33 amu
Does the following represent an element, compound, or mixture
(NH4)2SO4
compound
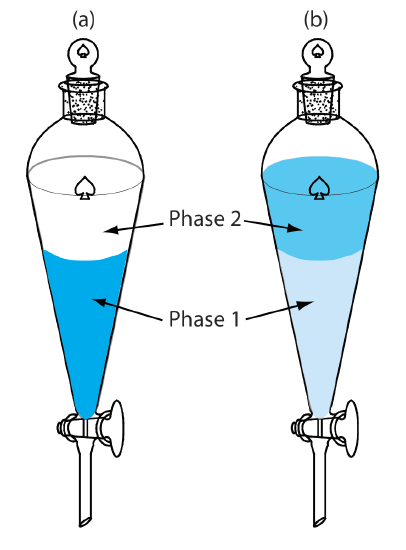
Extraction
Find the product(s) in the following chemical equation:
2 HgO(s) --> 2 Hg(l) + O2(g)
Hg and O2
Is the following a physical or chemical change:
Rain water evaporates from the pavement
Physical Change
What is the length of the object below?
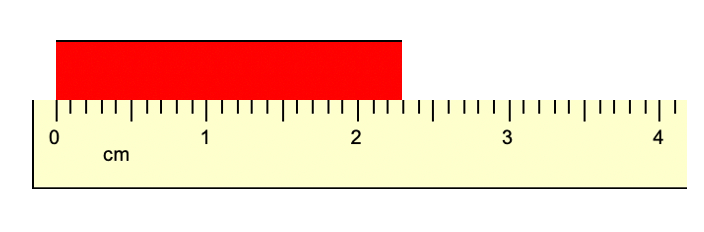
What is 2.29 cm?
or
What is 2.30 cm?
You are giving a patient 3.0 cm3/min of a given solution intravenously. If this is done for 24 hours, how many liters of this solution will you need?
4.3 L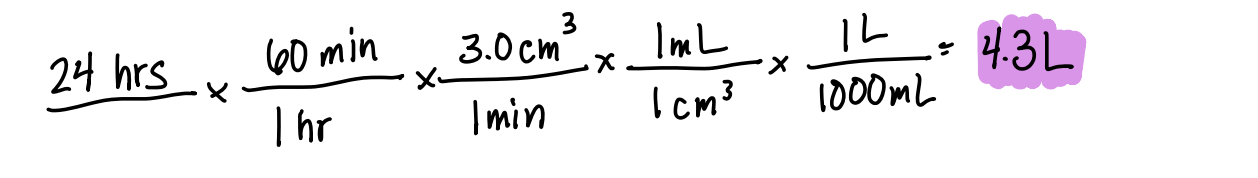
5.7621 m x 6.901 m x 0.460 m =
18.3 m3
A block of copper metal has a mass of 1896 g. The dimensions of the block are 8.40 cm x 5.45 cm x 4.6 cm. What is the density of copper?
9.0 g/cm3
Find the mass in 2.6 mol of lithium bromide (LiBr).
Is the following a physical or chemical property:
The freezing point of water is 0°C
Physical Property
sulfur
S8
A scientist is testing which temperature is best for mold growth. He places a sample of black mold in 4 petri dishes (small plastic dishes). He places each sample in a separate dark room and leaves them alone for 2 weeks. The first room is set at 30 degrees. The second room is set at 50 degrees. The third room is set at 70 degrees. The last room is set at 90 degrees. At the end of the 2 weeks, he counts the number of mold spores to see which sample grew the most. What type of variable best describes the statement below?
Which variable is the following: humidity of the room
extraneous variable
What is the element name for the element with the atomic number 11. What is its symbol?
name: sodium
symbol: Na
Does the picture below represent an element, compound, or mixture?
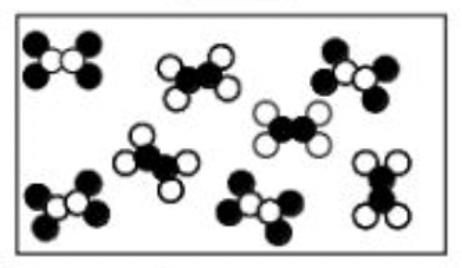
mixture
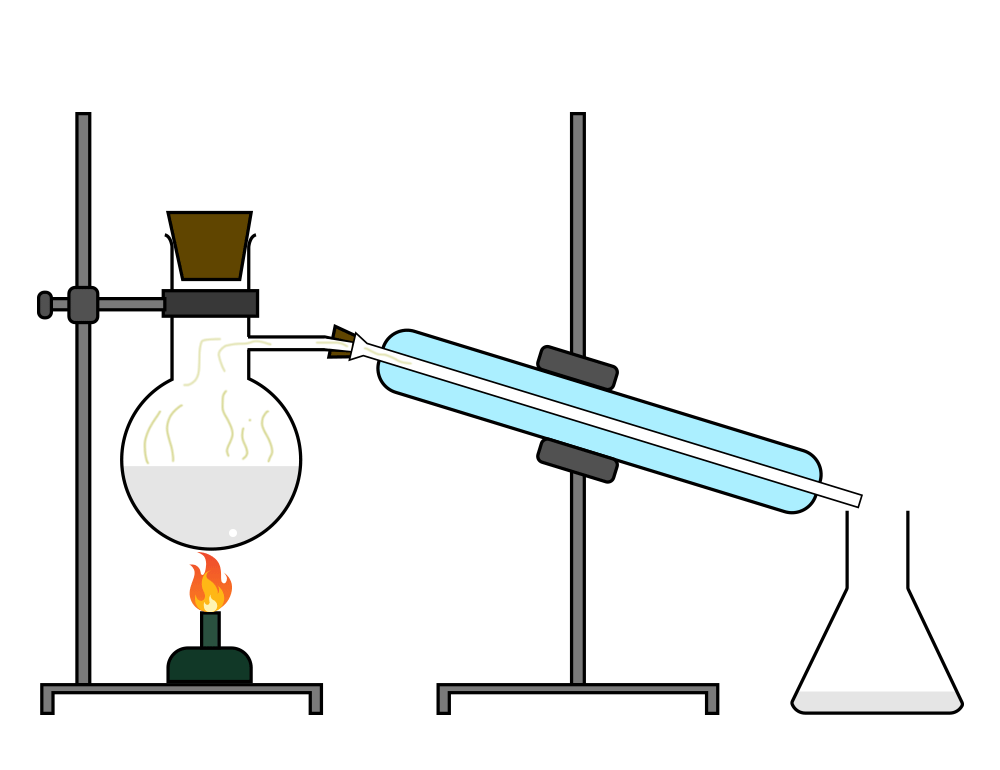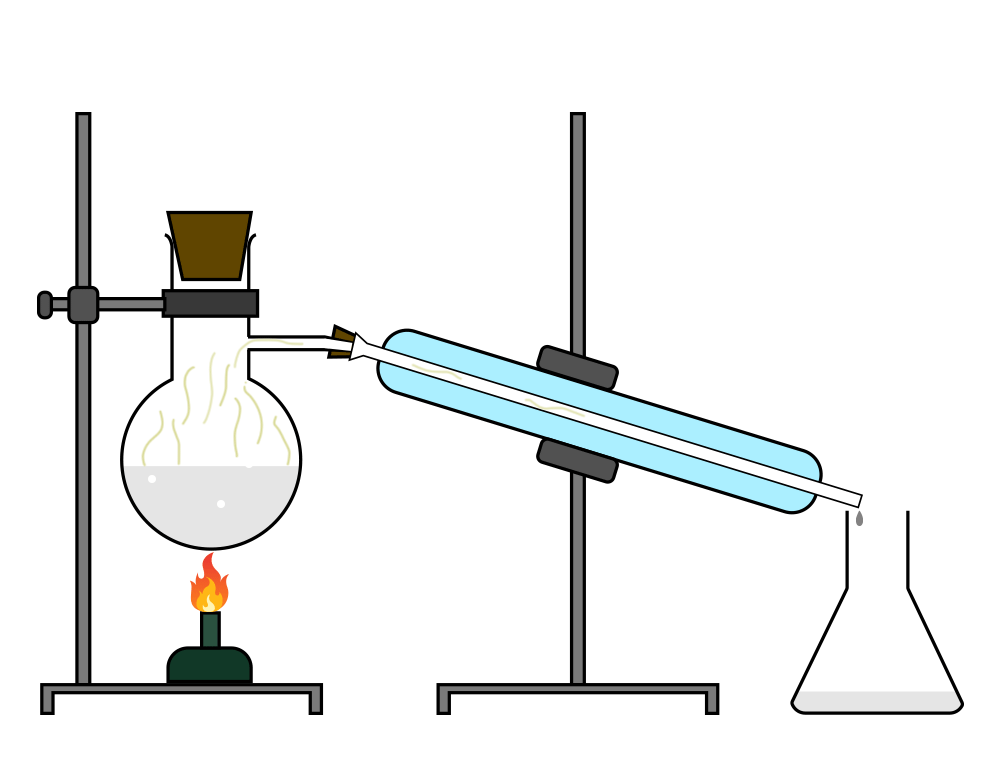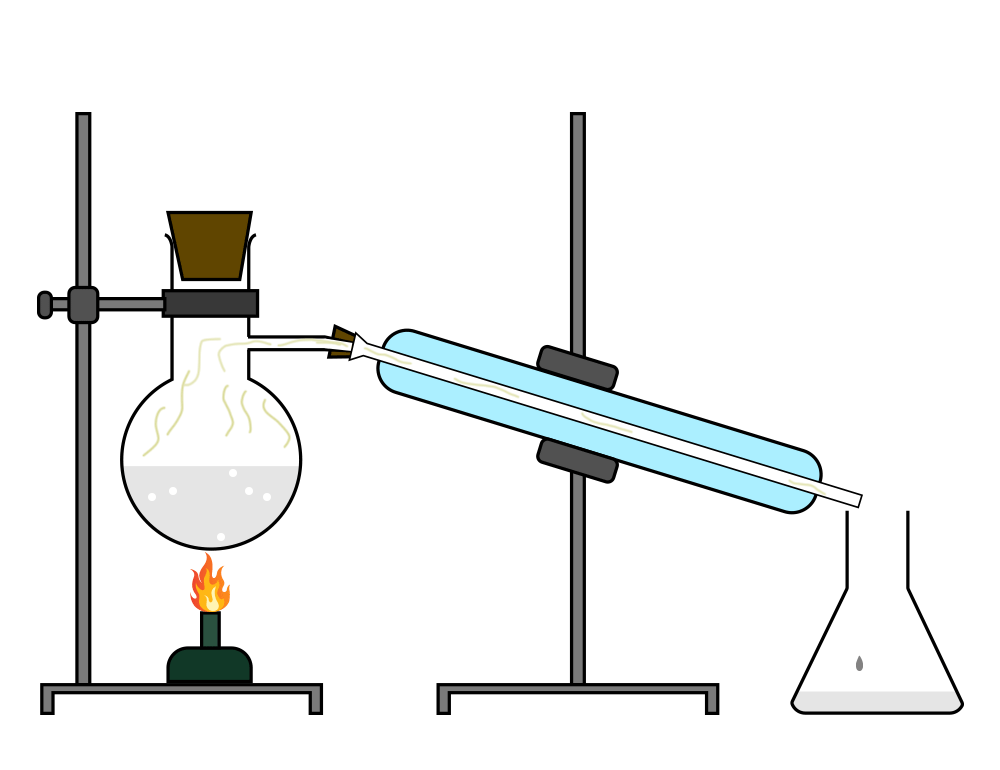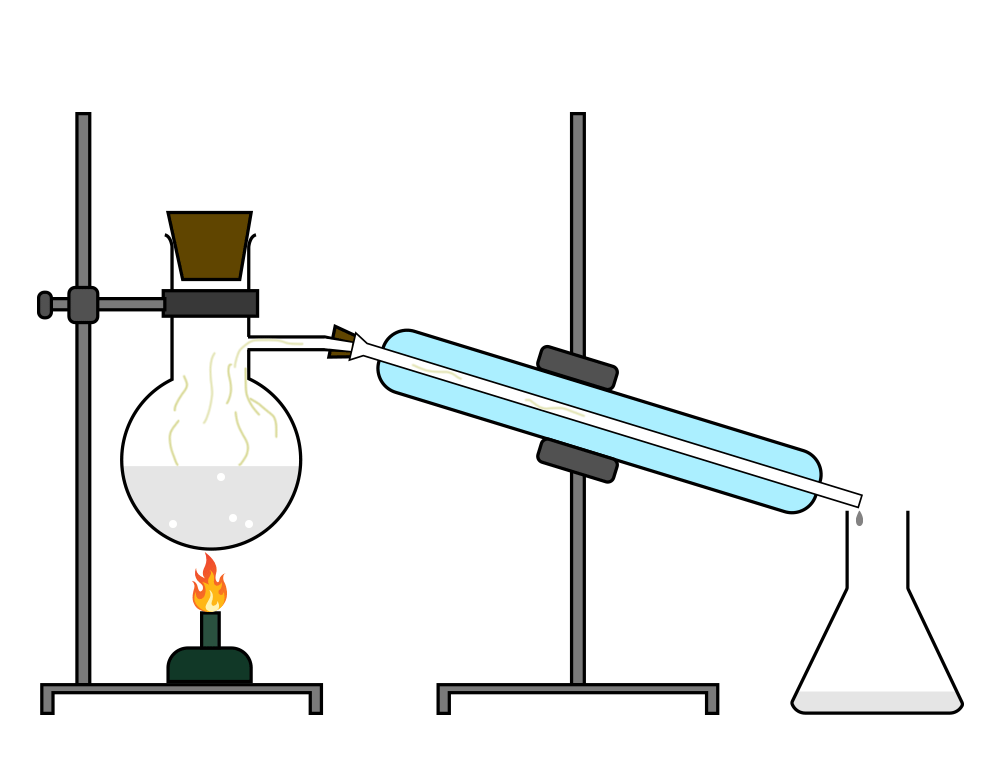
Distillation
Which substance(s) has a coefficient in the following chemical equation, what is the coefficient:
2 HgO(s) --> 2 Hg(l) + O2(g)
HgO and Hg
2
Is the following a physical or chemical change:
2NH3(g) ---> N2(g) + 3H2(g)
Chemical Change
Determine the volume of the liquid in the graduated cylinder.
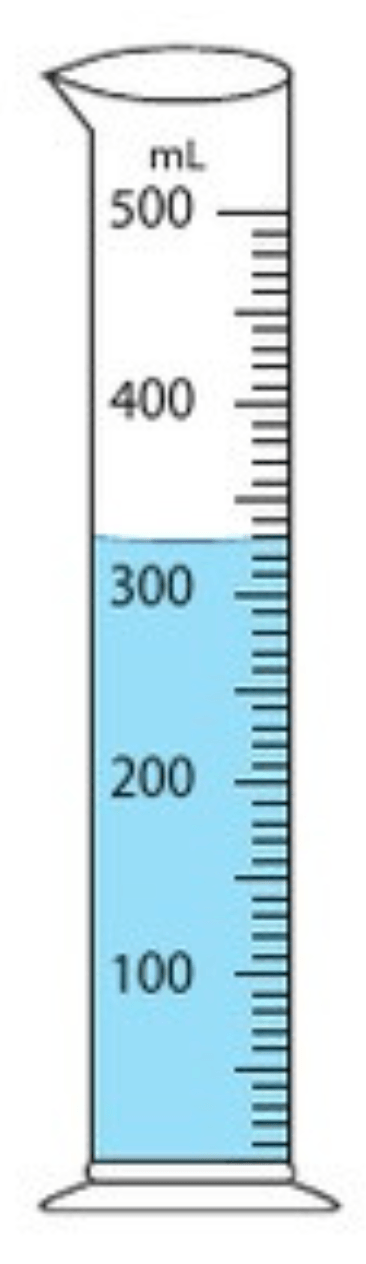
What is 328 mL?
(the range 326 - 330. would be acceptable)
You are buying carpet to cover a room that measures 39 ft by 40. ft. The carpet cost $18 per square yard. How much will the carpet cost?
$3100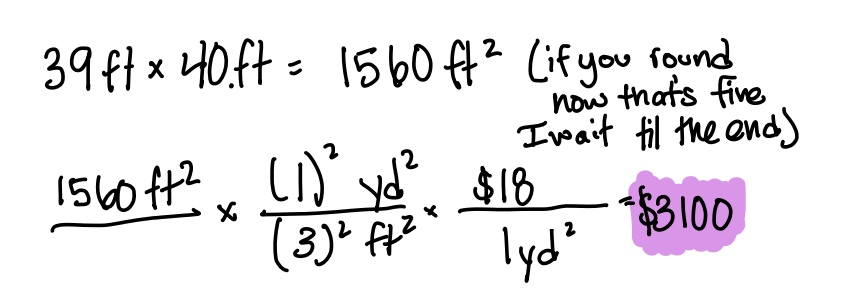
896.72 km + 1900 km = ??
What is 2800 km?
Aluminum can be sold to chemistry teachers in strips that are 5.00 cm wide and 0.40 cm thick. If a chemistry teacher needs a piece of aluminum that has a mass of 54.0 g, how long a piece would she need to cut? (density of aluminum 2.70 g/cm3)
l= 10. cm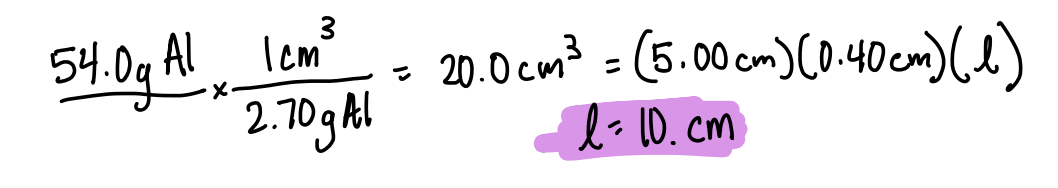
How many moles of magnesium is 3.01 x 1022 atoms of magnesium?
The percent error of a student who calculated the density of water as 0.98 g/mL when the actual density of water is 1.00 g/mL.
What is 2.0% error?
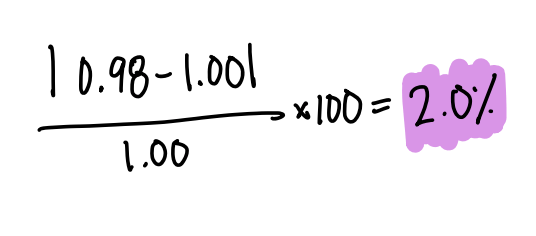
The 7 diatomic elements
Br2 I2 N2 Cl2 H2 O2 F2
Label the following statement as observation, hypothesis, experiment or scientific theory:
An object at rest will stay at rest, and an object in motion will stay in motion unless acted on by a net external force.
theory
In what period is uranium (#92) located?
Classify each of the boxes below as one of the following: pure element, pure compound, mixture of elements, mixture of compounds, mixture of elements and compounds
a.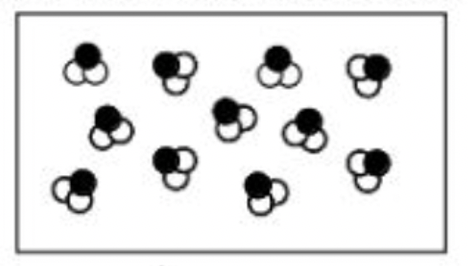
b. 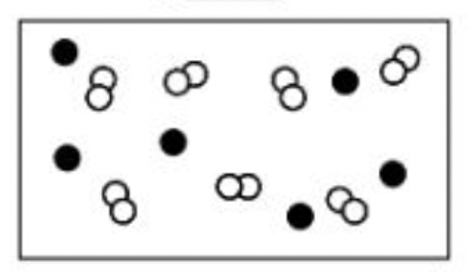
c.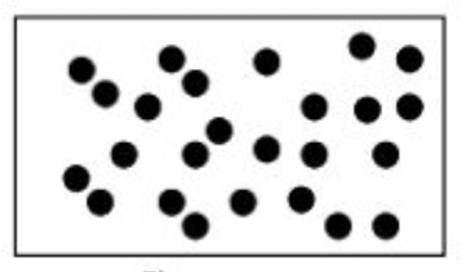
a. pure compound
b. mixture of elements
c. pure element

Sublimation
Which substance(s) has a subscript in the following chemical equation, what is the subscript:
2 HgO(s) --> 2 Hg(l) + O2(g)
O2
Is the following a physical or chemical change:
Physical Change
The temperature reading of the thermometer

What is 19°C?
(Since the thermometer increases by 2°C increments, can only be significant to the ones place)
A local zoning ordinance says that a house’s “footprint” (area of its ground floor) cannot occupy more than 25% of the lot it is built on. Suppose you own a 0.33 acre lot (1 acre = 43,560 ft2). What is the maximum allowed footprint for your house in square meters?
330 m2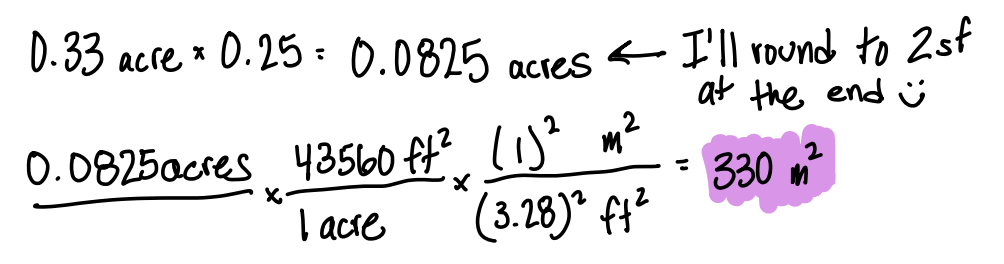
1.31 cm x 2.3 cm =
3.0 cm2
The annual production of sodium hydroxide (NaOH) in the United States in 2019 was 54.6 billion liters. The density of sodium hydroxide is 2.130 g/cm3, how many formula units (particles) of sodium hydroxide were produced?
1.75x1036 formula units NaOH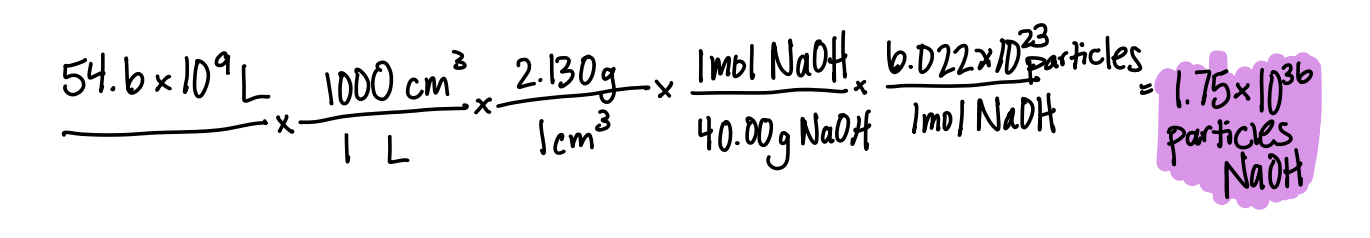
While cleaning a cut, you spill a bottle of iodine. The spill contains 1.40x1024 molecules of I2. What mass of the spill?
Explain why the mole is necessary.
The grouping atoms/molecules/ions that will relate the amount (number of particles) of a substance to its mass.
Allows chemists to mass particles in a laboratory
