(2.0000 - 1.00) / 2.00
What is 0.500?
Trail mix is an example of this.
What is heterogeneous mixture?
The element with 6 valence electrons and 2 orbitals.
What is oxygen?
Draw a Lewis Dot Diagram for chlorine
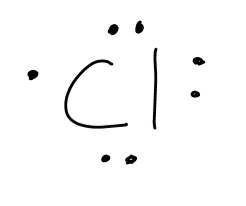
This is the name for this piece of equipment
What is a separatory funnel?
Convert 10 milliliters to liters.
What is 0.01 L?
This is an example of a chemical property
(Responses may vary - example: reactivity)
The metalloid with 3 valence electrons
What is Boron?
This element in group 14 has the smallest atomic radius
What is carbon?
In our measurement lab, how was liquid measurement recorded from the graduated cylinder?
What is from the bottom of the meniscus?
Convert 1.02 x 10^4 into standard notation.
What is 10200?

This is what is occurring at #4 on the graph
What is boiling?
The number of protons, neutrons and electrons in F- (Mass Number: 19).
What is 9 protons, 10 neutrons, 10 electrons?
Write the shorthand/noble gas configuration for Tin (Sn)
What is [Kr] 5s24d105p2?
In the spectrum lab, what kind of spectrum was produced from the helium lamp?
What is emission spectrum?
The mass of an object has a density of 2.7 g/mL. Find volume if the mass of the object is 12.8 g.
What is 4.7 mL?
A metal has a mass of 23.5 g, a specific heat of 0.288 J/goC, and temperature went from 25.0oC to 55.0oC. This is the energy.
What is -203 J?
This type of electromagnetic radiation is the most dangerous kind.
What is Gamma Rays?
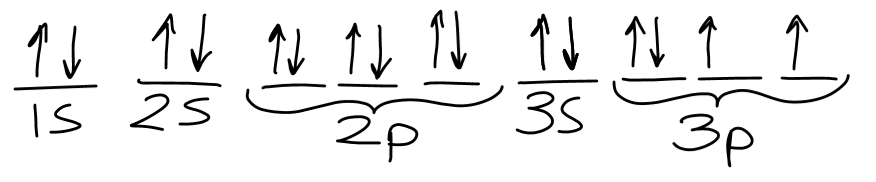
Draw the orbital filling diagram for Sulfur.
In the separation lab, this is the method of separation used for a heterogeneous mixture.
What is filtration?
A student measures the mass as 3.5 grams but the true value is 4.2 grams. This is the percent error.
What is 20%?
A student has a mixture of sand and salt dissolved in water. These are the methods of separation.
What is filtration then evaporation/distillation?
This is what happens to our energy level if we have a shorter wavelength.
What is higher/more energy?
Write the standard/longhand configuration for Argon (Ar)
What is 1s22s22p63s23p6?
In the calorie lab, this is the reason why percent error was (on average) high.
What is heat was not transferred completely, heat was lost to the surroundings.