What is the best way to separate the substances in a salt solution?
a. filtration c. evaporation
b. decantation d. sublimation
C. Evaporation
Who coined the term cell for the box-like structure he observed when viewing cork tissue?
a. Matthias Schleiden c. Rudolf Virchow
b. Theodor Schwann d. Robert Hooke
d. Robert Hooke
Which of the following is the fundamental quantity?
a. speed c. density
b. mass d. acceleration
b. mass
Distinct thread-like structures containing genetic information are called ____________.
chromosomes
In the formula C6H12O6, what do the numbers represent?
number of atoms
Which of the following is a scalar quantity?
a. velocity c. energy
b. acceleration d. force
c. energy
What is the name of the compound with a chemical symbol of NaHSO4?
Sodium Hydrogen Sulfate
Which of the following is NOT a paramagnetic atom?
a. Aluminum c. Iron
b. Helium d. Tungsten
B. Helium
Elements with paired electrons in their orbitals are said to be _____
Diamagnetic
Who founded the organizing principle of biology called evolution through natural selection?
Charles Darwin
Which of the following is NOT the correct SI unit?
a. mass:kg c. length: m
b. temperature: OC d. time: s
b. temperature: OC
Which of the following formulas represents the figure below?
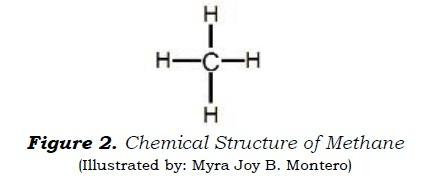
A. Empirical formula
B. Molecular formula
C. Structural formula
D. Condensed formula
C. Structural formula
for the multiple measurements, as the number of trials _________, the uncertainty ____________.
increases; decreases
This organelle is composed of different types of filaments. It also helps the cell transport materials
endoplasmic reticulum
Which of the following best describes Boyle’s Law?
- a) The volume of a gas is directly proportional to its temperature if the pressure is kept constant.
- b) The volume of a gas varies inversely with pressure, at a constant temperature.
- c) The pressure of a gas is directly proportional to its temperature, if the volume is kept constant.
- d) At constant volume and temperature, the total pressure of a gas is equal to the sum of its partial pressures.
- b) The volume of a gas varies inversely with pressure, at a constant temperature.
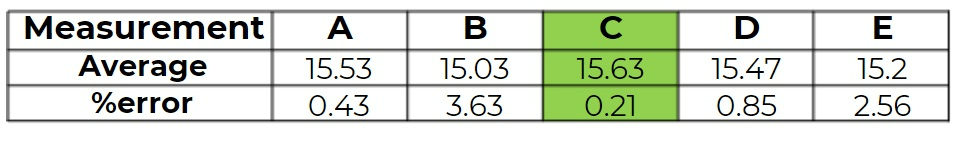
The standard mass of an unknown substance is 15.6 g. Which of the following sets of measurements has the most accurate average value?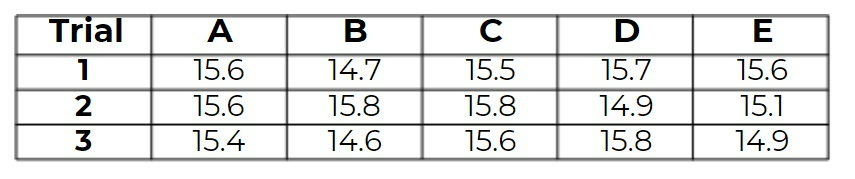
What stage in Meiosis does crossing over occur?
Prophase I
A science student read (NH4)2SO4 in a bottle. What is the name of this compound?
Ammonium sulfate