This is the beginning of the name of every ionic compound, with the sole exceptions being those that have positive polyatomic ions.
What is a metal / the metal atom?
This is the type of reaction that can occur between two aqueous solutions that contain ions.
What is double replacement reaction?
When Cu and FeSO4 are mixed together, will there be a reaction?
What is No?
Something dissolved in water is said to be in this state.
What is the aqueous state?
True or False: Something that goes from solid form to aqueous form has undergone a chemical change.
What is False?
[That is a physical change.]
True or False: You don't use prefixes when naming covalent compounds.
What is False?
This type of reaction has oxygen gas as a reactant.
What is combustion?
This is the most reactive metal, according to the Activity Series.
What is lithium?
An insoluble compound that falls out of a solution is given this label.
What is a precipitate?
OR what is a precipitant?
This is the name of a positive ion.
What is a cation?
True or False: This correct name for Mg(OH)₂ is magnesium (II) hydroxide.
What is False?
This is the opposite of synthesis (aka. combination).
What is decomposition?
This is the type of chemical reaction that the Activity Series is most helpful navigating.
What is single replacement?
This is the solubility of potassium chromate in water.
What is soluble?
This is the name of a class of ion that is made of multiple atoms, bonded covalently, that operates like a single unit in an ionic bond.
What is a polyatomic ion?
OR what are polyatomic ions?
This is the name of N2O4
What is dinitrogen tetroxide?
OR what is dinitrogen tetraoxide?
This is a major example of double replacement, often used to help clean and purify things.
What is neutralization?
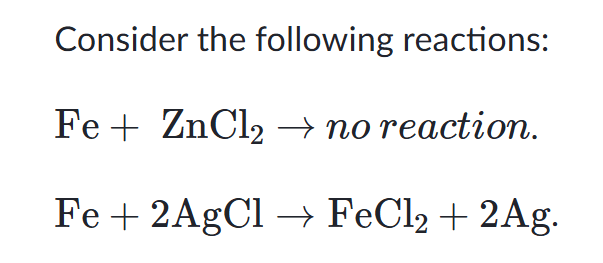
This is why the bottom reactionm occurs.
What is "iron is more reactive than silver?"
NaC2H3O2 is this degree of solubility.
What is soluble?
This is the formula for carbon diselenide.
What is CSe2?
This is the molecular formula for iron (II) oxide.
What is FeO?
This type of reaction requires an element & (separate from that element) a compound on the left side of the arrow, as well as a full reactionary exchange between the lone element and one of the elements in the compound.
What is single replacement?
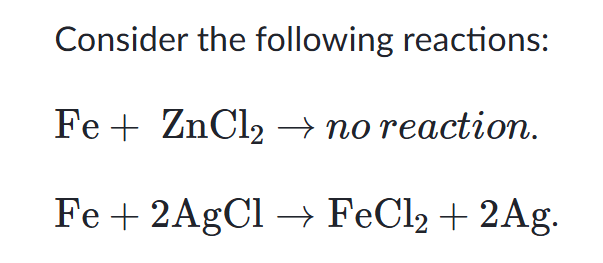
This means iron is _________ than zinc.
What is "iron is less reactive than zinc?"
Give an example of an insoluble compound.
(Answers will vary; double-check the exceptions column.)
If all identical bonds on a central atom of a molecule appear to have dipoles that cancel each other out, and there are no lone pairs on the central atom, this is the designation given to that molecule.
What is nonpolar?