The vast majority of any given atom is composed of this.
What is empty space?
This is the total charge of 5 electrons all by themselves.
What is -5?
OR
What is 5-?
Isotopes of the same element will have different amounts of __________.
What are neutrons?
OR
What is mass?
Almost all of the elements that touch the bolded staircase-shaped line on the Periodic Table are classified as this (other than solid).
What are metalloids?
OR
What is semimetal?
This is the formal name of Group 1 in the Periodic Table.
What are the alkali metals?
This is the functional purpose of neutrons.
What is "to keep the protons together in the nucleus"?
For practical purposes, this is what we consider the atomic mass of one electron to be.
What is 0 amu?
An ion is an element that has lost or gained electrons, typically to achieve stability in accordance with the ________ Rule.
What is the Octet (Rule)?
OR
What is the stable octet?
The group number of a representative element (i.e. an element that is NOT in the transition metals block) corresponds to the number of __________ in the ground state.
What are valence electrons?
This is the name of the inventor of the Periodic Table of the Elements as we know it today.
Who is Dimitri Mendeleev?
(Last name only is fine.)
The amount of this subatomic particle in an atom determines the element's identity, as well as many of its properties.
What is "protons"?
True or False: An electron can behave as both a wave and a particle.
What is True?
This is the atomic notation of an atom with 36e-, 39p, and 50n.
What is the following?
89 3+
Y
39
The period number of an element corresponds to this.
OR
What is the highest energy level?
What is the Bohr model?
OR
What is the planetary model?
When an atom is neutral (i.e. when it is not an anion or cation), these two totals must always be equal.
What are the number of protons and the number of electrons?
When an electron absorbs energy from its surroundings, this happens.
What is "the electron goes to a higher energy level"?
OR
What is "the electron jumps to a higher energy orbital"?
OR
What is "the electron enters an excited state"?
This is what the electron dot structure of a chloride ion looks like.
What is the following?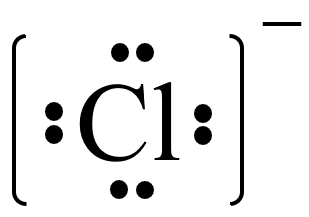 (For this game, allow credit for not including the - or brackets. That is new information. The key component here is the 8 electron dots.)
(For this game, allow credit for not including the - or brackets. That is new information. The key component here is the 8 electron dots.)
Moving diagonally from Fr to F, name one concept that will increase.
What is one of the following?:
Electronegativity, (First) Ionization Energy, Nonmetallic Character
Each shell can hold more electrons than the last. If the first shell can only hold 2 electrons max, this is the number of electrons the second shell can hold.
What is 8 electrons?
(Theoretically, the third shell can hold eighteen electrons max, which coincides with 18 groups.)
The dense, positive nucleus of an atom was first established with this atomic model. (Looking for the proper noun name, since the model can also be nicknamed the "nuclear model.")
What is the Rutherford Model?
If an electron in an excited state is at an energy level of n = 3, and it returns to its ground state at n = 1, this is what we would expect to be released from that electron.
What is (1 or 2) photon(s)?
This is what the Lewis structure of a carbon atom looks like.
What is the following?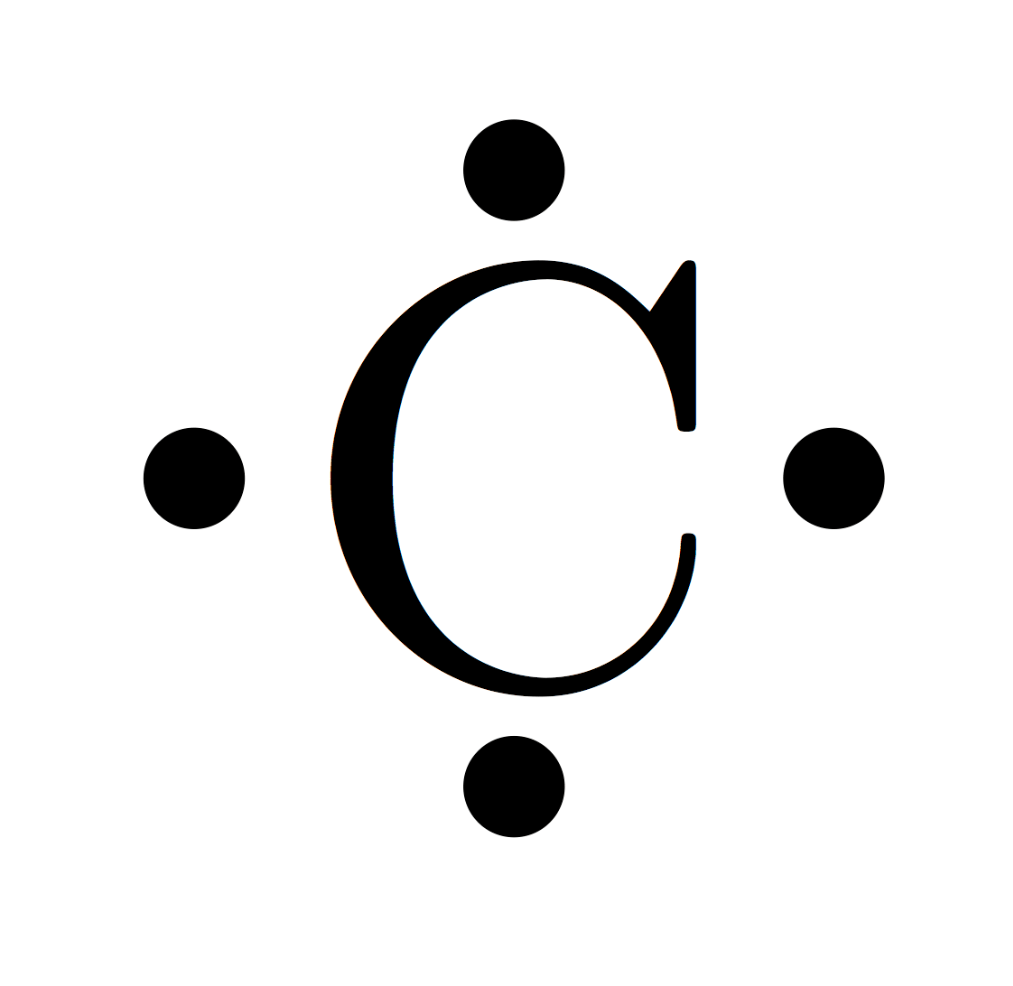
(No credit if any of the electron dots are paired up on a side or corner. That is NOT how to fill in a Lewis / electron dot diagram.)
Moving diagonally from Fr to F, name one concept that will decrease.
What is Atomic Radius / Atomic Size?
OR
What is Metallic Character?
If an element achieves a stable octet with its valence electrons, that means it has the same ____________ as the nearest noble gas.
What is electron configuration?
OR
What is number of valence electrons?