What is the name of a light particle?
Photon
Identify the element in period 3 that has the following ionization energies:
IE₁ = 787 kJ/mol
IE₂ = 1,577 kJ/mol
IE₃ = 3,232 kJ/mol
IE₄ = 4,357 kJ/mol
IE₅ = 16,000 kJ/mol
IE₆ = 19,400 kJ/mol
IE₇ = 23,300 kJ/mol
Si
Name at least 2 elements that break the octet rule other than hydrogen
Incomplete: Boron, aluminum, gallium, beryllium...
Expanded: Phosphorus, sulfur, chlorine, bromine, iodine, xenon, arsenic, selenium...
An affinity for electrons
Electronegativity
You need _____ to convert from volume to moles (and vice versa)
Molarity (concentration)
How many neutrons are in Carbon-12?
6
How tall am I?
6'0
What is the frequency of light if the energy of a photon is 8.64 ⋅ 10-14 J?
1.30 ⋅ 1020 Hz or s-1
What would be a possible set of quantum numbers for an electron?
o n = 2, l = 1, ml = -2, ms = -½
o n = 3, l = 4, ml = 0, ms = +½
o n = 3, l = 1, ml = -2, ms = -½
o n = 3, l = 2, ml = -2, ms = -½
n = 3, l = 2, ml = -2, ms = -½
What is the name of these 3 compounds?
NH4NO3
Ag3(PO4)2
PCl4
Ammonium Nitrate
Silver (II) Phosphate
Phosphorus Tetrachloride
A covalent bond where electrons are shared equally or almost equally.
Nonpolar bond
How much water must be added to 0.250 L of a 0.200 M NaCl solution to produce a 0.100 M NaCl solution?
0.25 Liters of Water
Note: Subtract original amount from the new amount of solution to obtain amount of water that's required to dilute solution.
How many sigma (σ) bonds and pi (π) bonds are there in the benzene molecule?
12 sigma bonds
3 pi bonds
Identify two people's majors at the table
nice
Which of the following transitions absorbs energy with the shortest wavelength?
o n = 1 → n = 5
o n = 7 → n = 1
o n = 1 → n = 7
o n = 6 → n = 2
n = 7 → n = 1
Write an electron configuration for an oxygen atom in an excited state.
NOT 1s22s22p4
Many correct answers
Note: There's a total of 8 electrons. Make sure the total amount of electrons in your configuration is 8.
Which of the following will form a strong electrolyte solution when dissolved in water?
o CaCl2
o KOH
o NH4F
o CH3OH
CaCl2 and KOH
Anytime you see Cl or OH dissolved in water, it will form strong electrolyte solution
This is the structure of retinol or vitamin A1. Determine the hybridization of the oxygen atom and the second nearest carbon to the oxygen atom.
Oxygen: sp3
Carbon: sp2
Potassium metal reacts with water as shown below. If 52.4 g of gaseous hydrogen were produced, how many grams of potassium were used?
2K (s) + 2H2O (l) → 2KOH (aq)+ H2 (g)
2033 g
A small amount of aqueous K3PO4 is added to a solution of CaS. What precipitate forms?
Ca3(PO4)2 (s)
What is the date and time of the final exam?
Monday (3/17) @7:30 AM!
What is the wavelength of light (in nm) emitted from a hydrogen atom when an electron in the atom excites from the n = 6 shell n = 3 shell?
1094 nm
Write the electron configuration of chromium (Cr)?
[Ar]4s¹3d⁵
What is the trend for an increase in the following: atomic radius, ionization energy, and electronegativity?
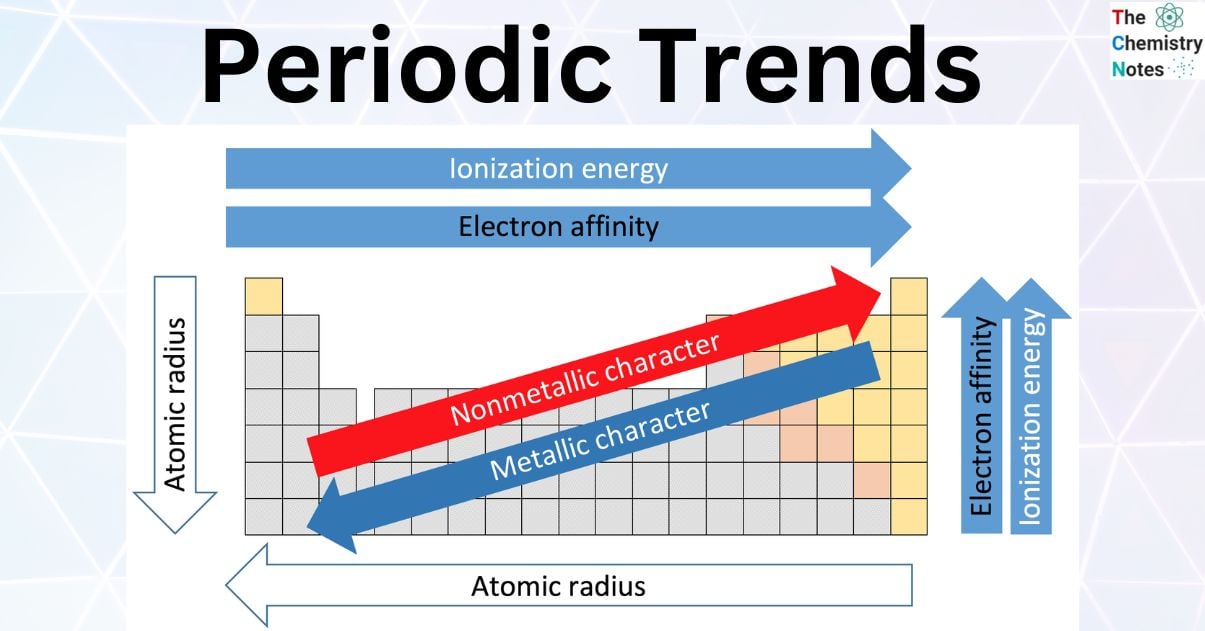
What is the best resonance structure for SNO-?
a) Balance the equation:
KOH (aq) + H2SO4 (aq) → K2SO4 (aq) + H2O (l)
b) How many mL of 0.5 M KOH is required to completely react with 250.0 mL of 0.50 M H2SO4?
2KOH (aq) + H2SO4 (aq) → K2SO4 (aq) + 2H2O (l)
500 mL KOH
What is the uncertainty in the position of an electron in an iron atom moving at 2.8 ⋅ 106 m/s with 8% uncertainty in velocity? Mass of an electron is 9.109 ⋅ 10-31 kg
2.58 ⋅ 10-10 m or 0.258 nm
Thomas Jefferson
Light strikes a metal surface causing the metal to emit electrons. The binding energy of the metal is 1.48 ⋅ 10-19 J and the kinetic energy of the electrons is 2.49 ⋅ 10-19 J. What is the wavelength of the light striking the metal surface?
500 nm
Write a possible set of quantum numbers for an electron in the 4d orbital.
n = 4
l = 2
ml = -2 to 2
ms = +½ or - ½
s = 0, p = 1, d = 2, f = 3 f
The 6 Strong Acids you MUST memorize
HClO4, HI, HBr, HCl, H2SO4, HNO3
Draw the correct lewis structure for NH3 and determine the following: electron geometry, molecular geometry, hybridization on the central atom, and the polarity of the molecule.
Electron Geometry: Tetrahedral
Molecular Geometry: Trigonal Pyramidal
Hybridization: sp3
Polarity: Polar
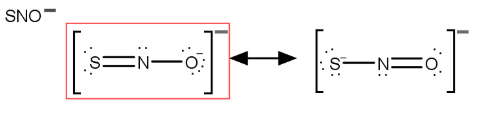
What is the ionic AND net ionic equation for this reaction?
Fe(CH3COO)3 (aq) + NH4OH (aq) --> ?
Magnesium has three naturally occurring isotopes. Determine the average atomic mass (AMU) of Mg-26.
24Mg (23.99 AMU, 79.0%)
25Mg (24.99 AMU, 10.0%)
26Mg
25.99 AMU
Finish the quote: "Oh we're so sorry to hear that your brother passed away he gets..."
5 big booms. BOOM BOOM BOOM BOOM BOOM
Light with a wavelength of 452 nm strikes a metal surface causing the metal to emit electrons. The velocity of one the electrons emitted is 5.97 ⋅ 105 m/s. What is the binding energy of the metal?
2.77 ⋅ 10-19 J
Name, define, and describe each quantum number.
Principal (n): Energy level of electron (n = 1+)
Angular Momentum (l): Shape of orbital (0 to n-1)
Magnetic (ml): Orientation of orbital (-l to +l)
Spin (mS): Spin of electron (+½ or -½)
Write the correct formula and charges for the following polyatomic ions:
Nitrate, sulfate, phosphate, hydroxide, acetate, carbonate, bicarbonate, ammonium, and cyanide.
NO3-, SO42-, PO43-, OH-, CH3COO-, CO32-, HCO3-, NH4+, CN-
Draw the correct lewis structure for SO2 and determine the following: electron geometry, molecular geometry, hybridization on the central atom, and the polarity of the molecule.
Electron Geometry: Trigonal Planar
Molecular Geometry: Bent
Hybridization: sp2
Polarity: Polar
For the reaction: Pb(NO3)2 (aq) + BaCl2 (aq) ---->
a) Identify the precipitate that forms and its mass when 200.0 mL of 0.25 M Pb(NO3)2 reacts with 300.0 mL of 0.20 M BaCl2.
b) What is the percent yield if a chemist produces 0.213 grams of the precipitate using the values above?
a) 13.9 g PbCl2
b) 1.5% yield
A compound containing only carbon and hydrogen is analyzed and found to be 84.13 % carbon by mass. The compound has a molar mass of about 114 g/mol. Determine the empirical and molecular formulae of this compound.
Empirical Formula: C4H9
Molecular Formula: C8H18
H2SO4(aq) + 2NaOH(aq) ----> Na2SO4(aq) + 2H2O(l)
a) What is the molarity of an H2SO4 solution if it requires 150 mL of 0.2 M NaOH to completely neutralize 50 mL of the acid?
b) Given your answer from part A, how much water must be added to dilute the H2SO4 solution to concentration of 0.05 M?
a) 0.3 M H2SO4 solution
b) 250 mL
Note: For part b, 300 mL was the total volume of the diluted solution. 250 mL of water must be added to the 50 mL of acid to reach a concentration of 0.05 M.