A state of matter that has no fixed shape or volume
Gas
A _______ is a state of matter with a fixed volume but no fixedshape.
What is a liquid?
temperature at which a substance changes from a liquid to a solid is called what
What is freezing point?
smallest basic unit of matter
what is an ATOM
A measure of the amount of matter present in a given volume of substance.
What is Density?
This is the measurement of the amount of matter in an object.
What is mass?
A _______ is a state of matter with a fixed shape and volume.
What is solid?
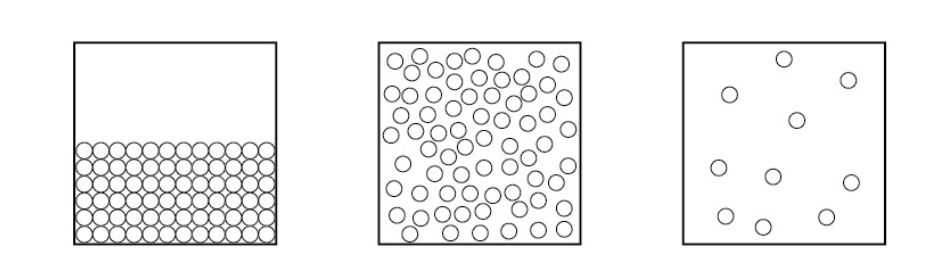
What are the 3 states in the PICTURE above
Solid , LIQUID , GAS
when two or more atoms bond together or combine
example H2O
what is an molecule?
Cutting paper, cutting vegetables, cutting cake are examples of.
What is a physical change?
anything that has mass and takes up space
What is matter?
Density describes the relationship between ____ and ____.
mass and volume
A liquid has a definite volume and has a definite shape.
TRUE OR FALSE
FALSE
A liquid has a definite volume but DOES NOT have a definite shape.
When a substance only has one thing in it it is called.
What is a Pure Substance?
What is the chart of elements called?
Periodic Table
the 5 senses we use in science are.
What are your 5 senses (hearing, seeing, touching, tasting, and smelling)?
a substance that only contains one type of atom
What is an Element?
The temperature at which a substance changes from a solid to liquid
What is melting point?
A substance that looks uniform (the same) throughout.
A substance that does not look the same throughout.
What is heterogenous?
A characteristic of a substance that can be observed or measured without changing its composition
What is physical property?
These are examples of what.
Oxygen, Carbon, Iron
What is an Element?
A change in the physical substance but not the actual substance itself
What is a Physical change?
These are examples of what.
Chex Mix, Soil, Italian Dressing
What are Heterogenous Mixtures?
These are examples of what.
Air, Tap water, Salt water
What are Homogenous Mixtures?