Is this a strong nucleophile, strong base, weak nucleophile, or weak base?
Br-
Strong base and strong nucleophile
(will react with alkyl halides to give either a mix of SN2 and E1 products if primary or secondary, E2 only if tertiary)
Which of these types of alkyl halides will react the fastest in an SN2 reaction?
A. Secondary
B. Tertiary
C. Methyl
D. Primary
C. Methyl
(methyl alkyl halides have the least amount of steric congestion for the backside attack step)
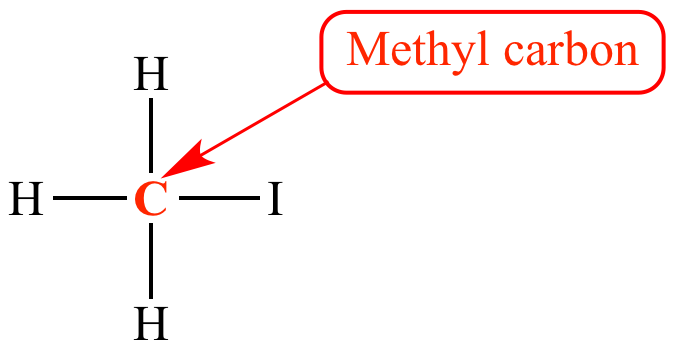
What is the correct order of events in an E2 reaction?
I. Loss of leaving group
II. Double bond is formed
III. Deprotonation of beta-carbons
A. I, II, III
B. III, II, I
C. II, III, I
D. III, I, II
D. III, I, II
(deprotonation of the beta carbons occurs first by the base, which triggers the loss of leaving group, allowing the molecule to form into an alkene)
Which reactions will produce both SN2 and E2 products?
(see options on white board)
Reaction 2 will produce both SN2 and E2 products
(strong base, strong nucleophile reacting with a secondary alkyl halide)
How many products will this molecule give when reacted with DBN? What type of alkene will it be?
1 product; Hofmann (DBN is a sterically hindered strong base/weak nucleophile, so it will not react to give a Zaitsev product due to steric hinderance)
With a tertiary alkyl halide, this reagent will produce what type of reaction?
OH-
SN1
(the bromide ion is a weak base/strong nucleophile so you would expect SN2, except we because the reagent is tertiary, it is SN1)
(keeps you on your toes)
Name the product of 1-bromo-1-methyl-cyclopentane when reacted with NaCN:
1-cyano-1-methyl-cyclopentane
(simple SN2 substitution of the bromine with the cyano group)
How is one able to obtain a major Hofmann product in an E2 reaction?
Use a sterically hindered, nonnucleophilic base to remove the beta proton
(using sterically hindered or "bulky" bases will not react to give a Zaitsev product, therefore only a Hofmann product can be formed)
Give two reagents that will produce an SN2 reaction and two that will produce an E2 reaction
Strong nucleophiles for SN2:
HO-, MeO-, EtO-, I-, Br-, Cl-, RS-, HS-, HSR-, H2S, etc.
Strong bases for E2:
DBU, DBN, HO-, MeO-, EtO-, etc.
Which compound will give an SN2 product when reacted with OH-?
1. 
2.
3.
#2 (it's on a secondary carbon, the rest are tertiary and will only give E2 products)