What are the 2 main ingredients in Oobleck?
water and cornstarch
a measure of a fluid's resistance to flow is called...
viscosity
With the right temperature and pressure, an element or compound can exist as a solid, a liquid, and a gas at the same time. What is this called?
triple point
The formula to find pressure is _______ divided by _________
force divided by area
The 3 temperature scales in science are Celsius, Fahrenheit, and...
Kelvin (based on absolute zero)
What's the difference between sodium metal and sodium chloride (table salt)?
sodium metal is an element, and sodium chloride is a compound
Vegetable soup, oil and water, soda with ice cubes are all examples of _________ mixtures
heterogeneous mixtures
Dry ice is solid carbon dioxide (CO2) If we apply heat, dry ice will go from solid to gas and never pass through the liquid phase. This is called...
sublimation
According to Boyle's law, a gas with a volume of 10L and a pressure of 4 Pa gets smooshed down into a new volume of 5 L. What is the new pressure?
8 Pa (if volume gets halved, then pressure is doubled)
The US Standard unit of pressure is PSI (pounds per square inch)
The metric unit of pressure is .....
Pascal (Pa)
Cubic, hexagonal, orthorhombic, and monoclinic are all examples of...
crystal shapes / crystal systems / crystalline solids
Coffee, milk, wine, and blood are all examples of _________________ mixtures
homogeneous mixtures
If the volume remains constant, a gas with increased pressure will always respond with increased temperature. This is an example of the gas law known as...
Gay-Lussac's Law
P1/T1 = P2/T2
This is the formula for...
Gay-Lussac's Law
The formula for calculating density is ______ divided by ________
mass divided by volume
What's the difference between the melting point and the freezing point of water?
It's the same thing 0 degrees Celsius or 32 degrees Fahrenheit
A device where we can pump the air out of a space decreasing the pressure down to zero is called a...
vacuum chamber
The Gas Law known as Charles's Law mathematically relates the 2 variables ____________ and _____________ in a direct proportion.
volume and temperature
According to Charles Law, a gas with volume of 10 mL and a temperature 5 C expands to 30 mL in volume. What is the new temperature of the gas now?
15 C
volume was tripled, so temp must triple
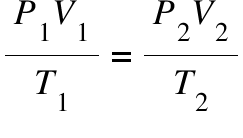
What is this?
combined gas law
Any solid can melt into a liquid. Any liquid can boil into a gas. Any gas can be ionized into a...
plasma (4th state of matter)
A scientific tool designed to measure air pressure is called a....
barometer
Boyle's Gas law states that as volume decreases, pressure increases. These 2 variables are in a mathematical relationship called...
inversely proportional
Answer all 3 questions:
What gases do gas laws apply to?
Where do gas laws work?
When have gas laws been observed failing to predict the correct answer?
Gas Laws apply to every gas, everytime, everywhere. They have never failed to predict the correct answer ever.
Palmyra is about 850 feet above sea level. This is known as the city's _____________
altitude or elevation