Without changing the mass of solvent or solute, provide three methods to increase the rate of dissolving a sugar cube in water.
increase temperature of the water
stirring or agitating the solution
increase surface area of the sugar
A lab group prepares a 0.500 L solution of 0.27 M KCl. They accidentally leave the solution uncovered overnight and return to find its volume decreased to 0.475 L. What is the new molarity of the KCl solution?
0.28 M KCl
Which of the following substances is likely to be most soluble in carbon tetrachloride?
a. HF
b. LiCl
c. NH3
d. Br2
d. Br2
Which of the following statements about aqueous solutions is NOT true?
a. in aqueous solutions, water is always the solvent
b. aqueous solutions are a type of homogeneous mixture
c. in aqueous solutions, the solute must be a solid
d. aqueous solutions are uniform throughout, with the solute evenly distributed in the solvent
c. in aqueous solutions, the solute must be a solid
the solute can be a solid, liquid, or gas
A solution of potassium chlorate has 20.0 grams of the salt dissolved in 100.0 grams of water at 70.0 degrees C. Approximately how many more grams of the salt can be added to the solution before reaching the saturation point?
10.0 grams
A 1.50 M sugar solution is prepared by mixing sucrose (C12H22O11) with enough water to create a 0.850 L solution. What mass of sucrose must be added to make this solution?
436 g
Which of the following substances is likely to be the most soluble in water?
a. I2
b. CBr4
c. C10H8
d. AlCl3
d. AlCl3
Which of the following solutions contains the greatest number of moles of NO3- ions?
a. 25 mL of 0.10 M KNO3
b. 10 mL of 0.15 M Ca(NO3)2
c. 20 mL of 0.20 M RbNO3
d. 10 mL of 0.10 M Sr(NO3)2
c. 20 mL of 0.20 M RbNO3
Category: What are you afraid of?
Nomophobia is a fear of
a. pregnancy
b. driving
c. numbers
d. being without your phone
e. the number 9
d. being without your phone
A beaker containing 80 grams of lead (II) nitrate in 100 grams of water has a temperature of 30 degrees Celsius. Approximately how many grams of salt are undissolved?
14 grams
Potassium permanganate is a strong oxidizing agent that is often used to treat water. It removes dissolved iron and manganese ions by converting them into insoluble forms that can eventually be filtered out. To test the efficiency of potassium permanganate in removing iron from groundwater, a chemist prepares a 2.25 L solution of 0.10 M potassium permanganate. What mass of potassium permanganate is needed to make this solution?
36 g
The structures of two compounds found in durian fruit, pantothenic acid (C9H17NO5) and capric acid (C10H20O2), are shown below.
Which compound is more soluble in water, and why?
a. pantothenic acid, because it contains a greater number of polar bonds than capric acid does
b. pantothenic acid, because it is less compact and has a larger surface area than capric acid does
c. capric acid, because it has a larger and more polarizable electron cloud than pantothenic acid does
d. capric acid, because it contains more carbon and hydrogen atoms than pantothenic acid does
a. pantothenic acid, because it contains a greater number of polar bonds than capric acid does
A beaker is filled with water and solute. It contains a membrane that is only permeable to water. Which of the following will occur?
a. the solute will move from side A to side B
b. water will move from side A to side B
c. water will move from side B to side A
d. the solute will move from side B to side A
c. water will move from side B to side A
Category: Basketball Players
Michael Jordan is a former professional basketball player. He played 15 seasons in the National Basketball Association (NBA) between 1984 and 2003, winning six NBA championships with the Chicago Bulls. Where did Michael Jordan go to university?
University of North Carolina at Chapel Hill

When 50 grams of potassium chloride are dissolved in 100 grams of water at 50 degrees Celsius, the solution can correctly be described as a(n) ______________ solution.
supersaturated
The figure shows two identical volumetric flasks containing the same solution at two different temperatures.
a. Does the molarity of the solution change with the change in temperature? Explain.
b. Does the molality of the solution change with the change in temperature. Explain.
a. yes - the molarity changes with a change in temperature. As temperature increases, volume increases, causing the molarity to decrease and vice versa.
b. no - molality does not change with change in temperature. Even though the volume has changed due to increased KE, the mass of the solute and solvent have not changed, therefore molality stays the same
What is thermal pollution? Provide a specific example explaining how thermal pollution harms the environment.
hot or cold water is dumped into a nearby body of water, changing its temperature
the decreased solubility of O2 in water as temperature increases is one of the effects of thermal pollution - fish may suffocate under these conditions
A student was asked to draw a diagram showing the interactions of the different particles present in an aqueous solution of calcium chloride. Their work is shown below. What two errors are present in the student's diagram?
a. the depicted ratio of Ca2+ to Cl- ions is inconsistent with the chemical formula of calcium chloride
b. two of the water molecules surrounding the Ca2+ ion are drawn in the wrong orientation
c. all of the water molecules surrounding the Cl- ions are drawn in the wrong orientation
d. the diagram indicates that the Ca2+ ion is larger than the Cl- ion, but the reverse is true
c. all of the water molecules surrounding the Cl- ions are drawn in the wrong orientation
d. the diagram indicates that the Ca2+ ion is larger than the Cl- ion, but the reverse is true
Name the song and the artist.
Abracadabra by Lady Gaga
Gases are most soluble in liquids at
a. high temperature and low pressure
b. low temperature and low pressure
c. high temperature and high pressure
d. low temperature and high pressure
d. low temperature and high pressure
What is the percent by mass of sodium bicarbonate (also known as sodium hydrogen carbonate) in a solution containing 20.0 g of sodium bicarbonate dissolved in 600.0 g of H2O?
3.23%
The structures of vitamins E and B6 are shown below. Predict which is largely water soluble and which is largely fat soluble. Explain. (white = hydrogen, black = carbon, blue = nitrogen, red = oxygen)
Vitamin B6 is likely to be largely water soluble. The three -OH groups and the nitrogen can enter into many hydrogen bonding interactions with water
Vitamin E is likely to be largely fat soluble. The long, rod-like hydrocarbon chain will lead to stronger dispersion forces among vitamin E and the mostly nonpolar fats. Although vitamin E has one -OH and one O group, the long hydrocarbon chain prevents water from surrounding and separating the vitamin E molecules, reducing its water solubility
What is the molality of a solution containing 10.0 g of sodium sulfate in 1000.0 g of water?
0.0704 m
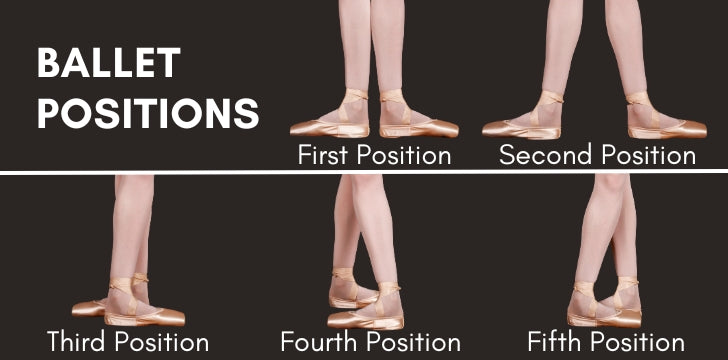
Category: Ballet
In which ballet position do the feet NOT touch each other?
a. first
b. fifth
c. fourth
d. third
c. fourth