What could you do to SLOW DOWN dissolving?
Remove heat, use larger pieces, stop stirring
What type of solution is represented by the point on the curve?
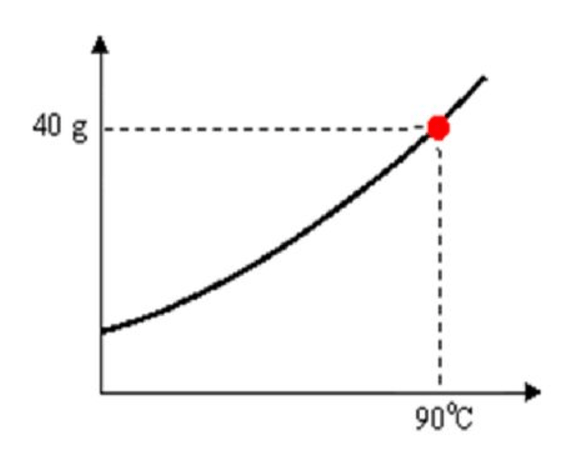
Saturated solution
How do you know a substance dissolved in water is an electrolyte?
Conducts electricity!
Which substance is closest to neutral?
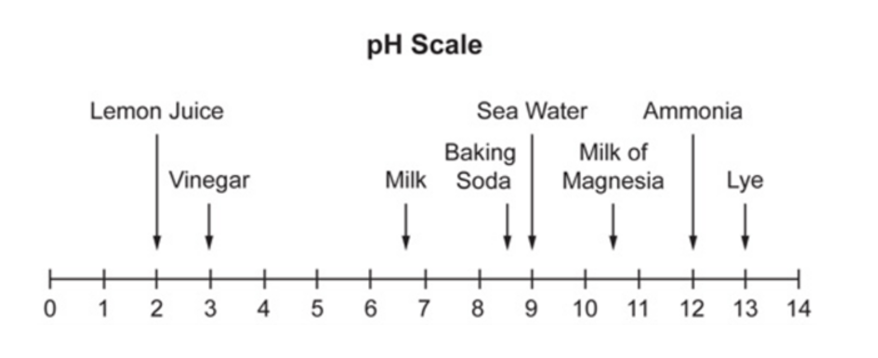
Milk
Acids turn litmus paper ________, bases turn litmus paper ________
Acids- RED
Bases- BLUE
What THREE factors increase the solubility of a solid?
Stirring, heat, and small size!
What is happening to the solubility of all three solids as temperature increases?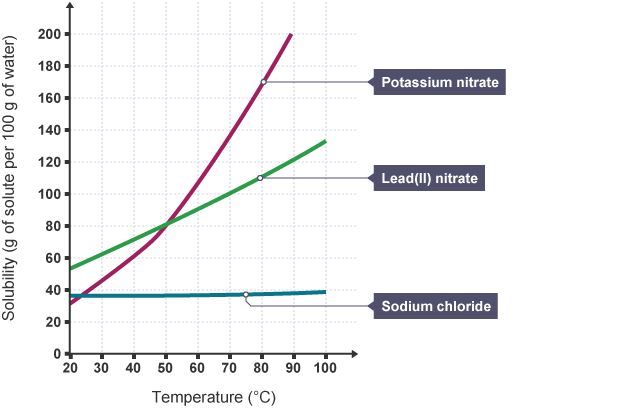
Solubility INCREASES with temperature
Which beaker would dissolve a solid the fastest?
Beaker A: 30°C
Beaker B: 40°C
Beaker C: 50°C
Beaker D: 60°C
Give an example of a possible pH of a BASE
Anything over 7!
What is used to determine the pH of a substance?
Indicator
Which solution has the highest salt concentration?
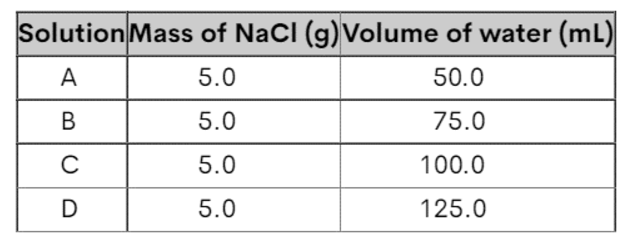
A
Which point represents a solution with too much solute for the given temperature?
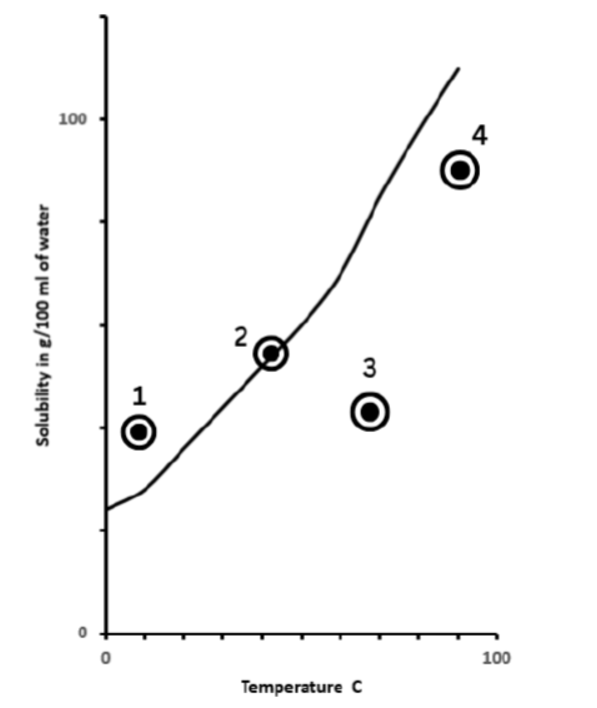
1
A solution in which MORE solute can be added
UNSATURATED
Give an example of a possible pH of an ACID
Anything below 7
Give an example of a FORMULA for a compound that is a BASE
NaOH
(Anything that ends with OH)
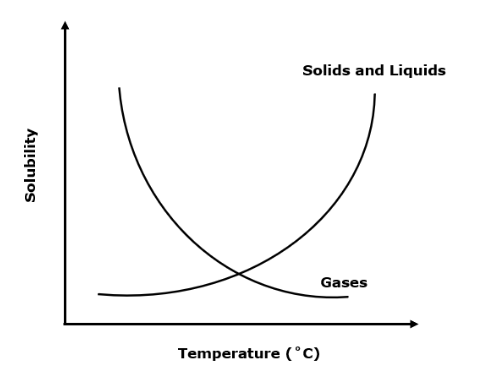
Create a solubility graph with one line for SOLIDS and one line for GASES:
What is the solubility of sugar at 20 degrees Celsius?
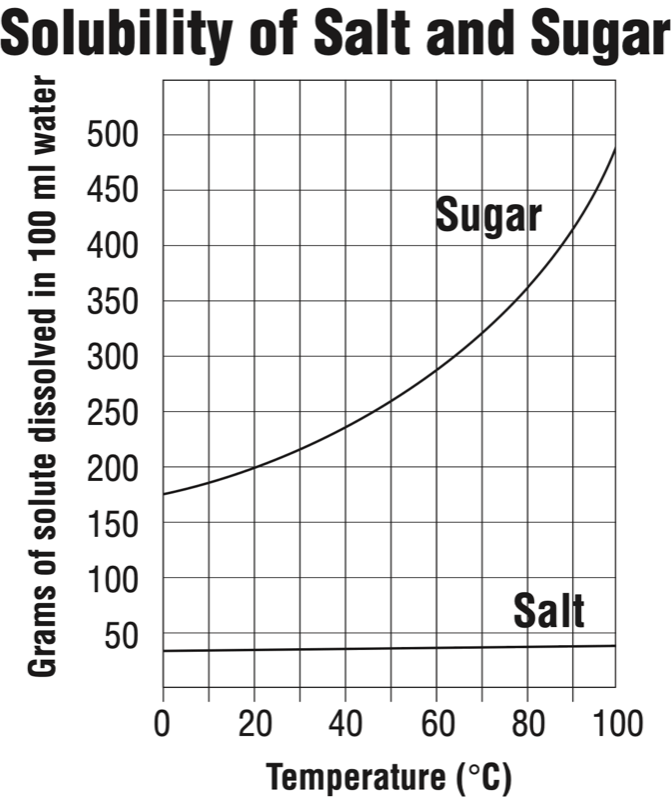
200 grams
Given a small amount of salt water, what TWO things can you do to make the salt water safer to drink?
Filter out the salt OR add a lot more water
What would be a possible pH for a WEAK base?
7.5 - 8.5
What would you use to neutralize a strong acid?
A (strong) base
What TWO substances have the SAME solubility at the same temperature?
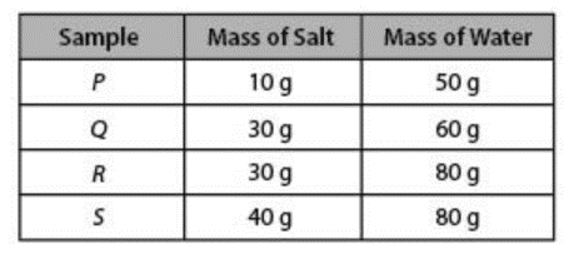
Q & S
Which solute has the greatest solubility as temperature increases?
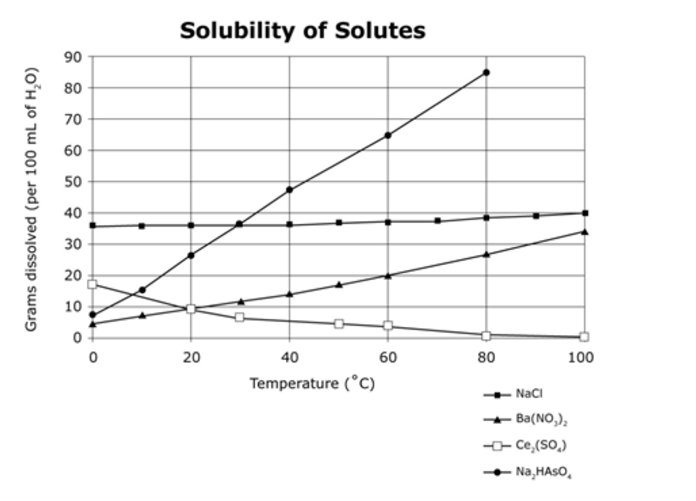
Na2HAsO4
Given a specific amount of solute and solvent, what TWO things could you do to increase the concentration of a solution?
INCREASE solute
DECREASE solvent
In order to neutralize acidic soil, you should look for a fertilizer with 1) what kind of ions to 2) raise or lower the pH?
OH- ions to RAISE the pH of the soil!
If a solution turns litmus paper red, what kind of ions does it release in solution?
H+