a substance made of one type of atom
Element
What are Reactants?
the substance or substances that go into a chemical reaction.
Physical or Chemical Change?

physical
the atoms are in the same order
How many Hydrogen Atoms are in H2O?
2
Zn + S -> ZnS
Is this Equation Balanced?
Yes
a mixture of two or more substances that appears to be a single, uniform medium, with no visible separation between its components
homogeneous mixture
the word for substances that come out of a chemical reaction.
Products
Physical or Chemical Change?
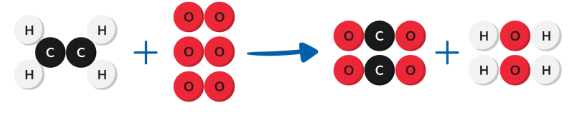
Chemical
the atoms rearranged and formed new substances
How many Hydrogen atoms are in 2NH4?
8
Balanced or Unbalanced? Why?
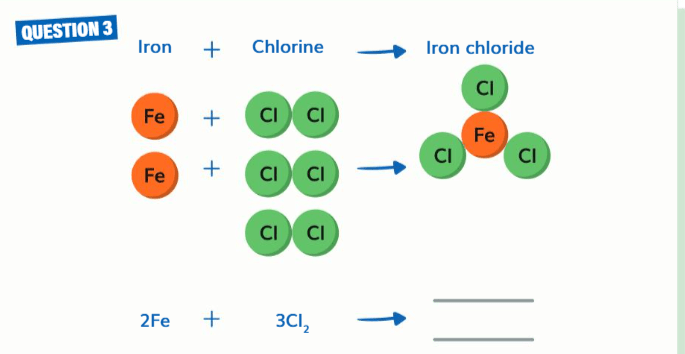
unbalanced
need to double the "Iron Chloride"
the amount of matter in an object, measured in kilograms
mass
Rearrange
Is RUST an example of a physical or chemical change?

Chemical
Rust shows the reaction between metal water and air

What is the correct formula for these atoms?
3Cl2
Balanced or Unbalanced? Why?
Unbalanced
you need to 4x the water
a chemical change that occurs when two or more substances combine to form a new substance
Chemical Reaction
an equation where there are different numbers of atoms on either side
unbalanced chemical equation
Why is it impossible to turn a copper penny into gold or silver?
based on the law of conservation of matter, what goes in must come out. If gold does not go into the chemical reaction, then gold cannot come out. one element can never turn into another one.
Draw a model for the compound FeCl3

_H2+_N2->2NH3
How would you balance this equation?
3H2+_N2->2NH3
the extent to which a material dissolves in another substance to make a solution, like sugar in water
solubility
The Law of Conservation of Matter:
Matter cannot be ______ or _______, it can only _______ forms.
Matter cannot be created or destroyed, it can only change forms.
What are the 6 signs of chemical reaction?
Change in color, Change in Temp., Change in Odor, Formation of a precipitate, formation of gas, production of light/sound
What is the correct formula for these atoms?
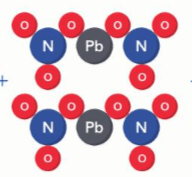
2PbN2O6
How do you balance the equation....
H2 + O2 -> H2O
2H2 + O2 -> 2H2O