What is the mass of 5 moles Ca(NO3)2?
820 g Why?
5 moles x 164 g/mol = 820 g
Is H2CO3 a strong acid, strong base, weak acid, weak base, or neutral salt?
weak acid
What charge does each oxygen atom become when substances go through combustion?
Alkali Metals
Li, Na, K, Rb, Cs, etc.
Also, NH4+
If the surrounding temperature increases, then the reaction is ( endothermic / exothermic ).
exothermic
What are 2 things you can do to slow down a reaction?
cool it
dilute it (lower concentration)
? H2+1 O2-->2 H2O
What is the coefficient of H2?
2
How many moles are 300 Ar atoms?
5 x 10-22 moles Why?
300 atoms / (6 x 1023 atoms/mole) = 5 x 10-22
What is the pH of distilled water?
7 Why?
Distilled Water is pure H2O which is neutral.
loses 2
Is BaSO4 soluble or insoluble in water?
insoluble
endothermic
more
_Al +_NaCl -->_AlCl3 +_Na
Write the balanced chemical equation.
1 Al + 3 NaCl --> 1 AlCl3 + 3 Na
4 Al + 3 O2 --> 2 Al2O3
How many moles of O2 gas react with 216 g Al?
6 moles O2 Why?
216 g Al / (27 g/mol) = 8 moles Al
8 moles Al is 2x more than 4
so moles O2 will be 2x more than 3.
Write the chemical equation for the dissociation of HCN in water.
HCN + H2O <----> H3O+ + CN-
K + NaCl --> KCl + Na
Which element went through reduction?
Na Why?
It's charge decreased from +1 to 0.
CaCl2+AgNO3-->Ca(NO3)2+AgCl
What is the precipitate?
AgCl
4 Fe + 3 O2 --> 2 Fe2O3 + 822 kJ
Is this reaction endothermic or exothermic?
exothermic
What are 3 things that could increase the rate of a reaction.
heat it
increase concentration (add solute, evaporate water)
add catalyst
___ CaCl2 + ___ K3PO4 --> ___ KCl + ___ Ca3(PO4)2
What are the coefficients of this chemical equation when it is balanced? Write them in order, separated by commas.
4 mL of 0.05 M KOH was added to 20 mL of HCl until a color change was seen. What was the initial pH of the original HCl solution?
3
(1) Add phenolphthalein to KOH until a color change is seen.
(2) Add HCl to that solution until a color change is seen.
Clear --> Pink
Pink --> Clear
4 K + O2 --> 2 K2O
Which element went through reduction?
Oxygen
Why? charge decreased from 0 to -2
What is the precipitate when K3PO4 is mixed with MgCl2?
Mg3(PO4)2
Is this describing an endothermic or exothermic reaction?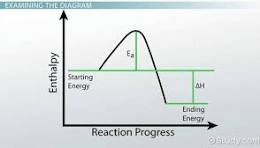
Exothermic Why?
PE of reacting chemicals decreased.
The amount PE decreased is the amount of KE that increased, warming up the surroundings.
when reacting particles are closer together, they take less time to collide.
___ P + ___ O2 --> ___ P2O5
Balance this equation.
4 P + 5 O2 --> 2 P2O5
How many grams of NaOH must be added to 5 Liters of water to make its pH 12.
2 g Why?
pH = 12 --> pOH = 2 --> [OH-] = 0.01 M
(M) (L) = moles --> (0.01)(5) = 0.05 moles
0.05 moles x 40 g/mol = 2 g
If equal moles of HCl and NiOH were mixed together, the resulting solution would have a pH ? 7.
>, <, or = ?
<
Why? HCl is a strong acid; NiOH is a weak base, the product is a weakly acidic salt, making the pH < 7.
K + MgCl2 -->2 KCl + Mg
Write the oxidation half reaction.
Mg+2 + 2e-1 --> Mg
What 2 aqueous solutions could be mixed together to form a CaCO3 precipitate?
Ca(NO3)2 or CaCl2
with
X2CO3 if x = Li, Na, K, Rb, Cs, or NH4
4 Fe + 3 O2 --> 2 Fe2O3 + 822 kJ
How much heat will be released when 111.7 g of Fe metal reacts with oxygen gas?
411 kJ Why?
111.7 g / (55.85 g/mol) = 2 moles
4 moles Fe release 822 kJ
half that number of moles will release half the kJ
Use collision theory to explain WHY increasing the temperature of a solution makes the reaction occur faster.
higher temp = faster motion = less time to collide
also
faster motion = more force during collision = higher % of collisions occur with enough energy to break bonds (KE >or= Activation Energy)
C4H10 + O2 --> CO2 + H2O
What is the coefficient of O2 when the equation is balanced?
13 .... Why?
2 C4H10 + 13 O2 --> 8 CO2 + 10 H2O