7.0
What is the currency for Massfield?
A Precipitate is in the ____________ phase of matter
solid
what is matter?
matter is anything that takes up space and has mass
What is the only disturbance that has an affect on Keq?
Temperature
During Chemical Equilibrium; what is the same?
What are the three "towns" on the stoichiometry roadmap?
Massfield, Moletown, Atomville
In an Ionic Compound, the Cation’s charge plus the Anion’s charge must add up to equal ________.
o
what happens if you put a less dense and a more dense liquid in a container?
the less dense liquid will float on the more dense liquid
The reaction shifts to the right; toward the products.
How do concentrated and dilute solutions look at the molecular level?
Concentrated: Lots of solute in a small amount of solvent
Dilute: Small amount of solute in a large amount of solvent
How do you calculate Molar Mass of a substance?
True or False? Iodine Chloride (ICl) is a Dipole because it has 2 poles, one end with a Partial Positive Charge and the other end with a Partial Negative Charge.
true
what is a physical/chemical change
physical changes is a change in size or shape and no new substance is formed
a chemical change is a change in the physical and chemical properties and a new substance is formed
for the rxn below explain how lower[HCO3^-1] impacts shell (CaCO3) formation
Ca^+2(aq)+2HCO3^-1(aq)=CaCO3(s)+CO2(g)+H2O(L)
is [HCO2^-1] goes down the shell (CaCO3) formation also goes down
1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12+13+14+15+16+17+18+19+20+21+22+23+24+25+26+27+28+29+30+1+2+3+4+5+6+7+8+9+10+11+12/0
0
Calculate #mol Al need to react with 9.9 mol MgCl2:
3MgCl2(aq) +2Al(s) -> 3Mg(s) +2AlCl3(aq)
6.6 mol Al
Why is Ammonia (NH3) considered Polar?
Because the Bond Polarities combine to give one end of the molecule a Partial Positive Charge and the other end a Partial Negative Charge.
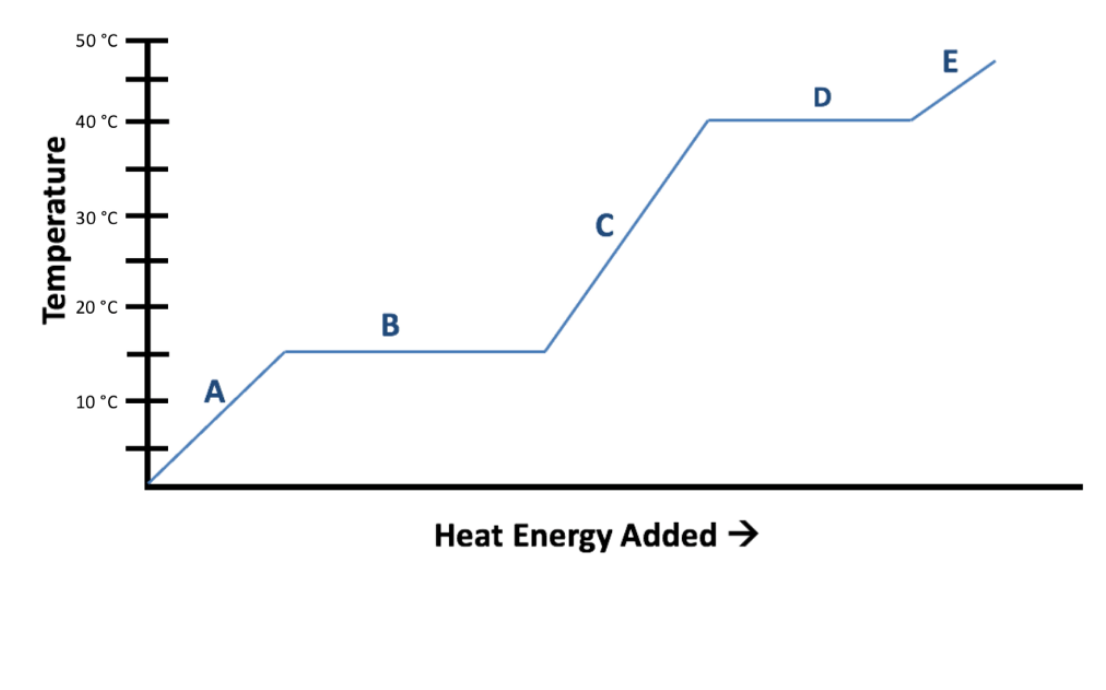
what happens at each point on the graph of temp of water
a is solid
b is solid/liquid
c is liquid
d is liquid/gas
e is gas
Draw/Complete chart for reaction:
H2(g) + I2(g) ⇌ 2HI(g) ∆=52 kJ
Increase [HI]
Left Shift; Up Arrow; Up Arrow; N/A; N/A
HCN (aq) + OH^-1 (aq) ⇋ H2O (l) + CN^-1 (aq)
Determine Acids and Bases and Pairs for this reaction
HCN (aq): BLA ; OH^-1 (aq): BLB ; H2O (l): CA ; CN^-1 (aq): CB
HCN / CN: ACBP
OH / H2O: BCAP
Determine Limiting Reactant and Excess Reactant then calculate #g NaCl produced in 59.6g FeCl2 reacts with 36.8g Na3Po4.
41.9g NaCl
Why is Carbon Tetrachloride (CCl4) considered Nonpolar?
Because the Bond Polarities extend equally and symmetrically in different directions, canceling each other's effect.
how many moles of gas are in a 30.0 L scuba canister if the temperature of the canister is 26.85 degree sign C and the pressure is 208 atm?
253 mol