What is the molar mass of Iron (Fe)?
What is 55.845 g/mol
A solution that contains less solute particles than are needed to form a saturated solution is _____.
What is unsaturated
HCl solution would have a pH greater than 7?
True or False
What is False
Stored energy due to the position of an object.
What is potential energy
Balance the chemical equation and give the coefficients for each compound.
____ Fe + ____ O2 → ____Fe2O3
What is 4:3:2
What is the correct formula for Potassium oxide?
What is K2O
Which of the following is the correct Lewis Dot structure for a chloride molecule, Cl2?
What is 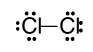
What is the molar mass of Mg3N2?
What is 173.844 g/mol
A solution which cannot dissolve any more solute without raising the temperature is called
What is saturated
A strong base is a substance which
What is dissociates (ionizes) completely in solution
How does heat transfer work with convection?
What is movement in fluids(liquid or gas), like a current. Heat rises, pushing cold down.
Consider the following chemical reaction. What is the mole ratio of hydrochloric acid to magnesium chloride?
1Mg(s) + 2HCl(aq) → 1MgCl2(aq) + 1H2(g)
What is 2:1
What is the formula for Disulfur decafluoride?
What is S2F10
Why can gases be greatly compressed?
What is molecules of gases are spaced far apart from each other.
What is the molar mass of calcium hydroxide - Ca(OH)2?
What is 74.092 g/mol
A solution which has water as the solvent is what kind of solution?
What is aqueous
State whether the solution of vinegar (HC2H3O2) is acidic, basic, or neutral if it has the following hydrogen concentration.
[H+] = 1.0 x 10 -6 M
What is pH = 6, acidic
When ice is added to a cup of hot tea, which statement is correct?
What is heat energy travels from the tea to the ice, causing the ice to melt
Which of the following equations represents a precipitate reaction?
1. LiOH(aq) + HBr(aq) → LiBr(aq) + H2O(l)
2. 2HCl(aq) + Zn(s) → ZnCl2(aq) + H2(g)
3. H2CO3(s) → H2O(l) + CO2(g)
4. KI(aq) + AgNO3(aq) → KNO3(aq) + AgI(s)
What is #4 reaction
What is the formula for Aluminum acetate?
What is Al(C2H3O2)3
The energy required to start a reaction is called
What is activation energy
What is the molar mass for Li3PO4?
What is 115.793 g/mol
Copper(II) chloride, CuCl2 is insoluble in water
True or False
What is False
According to the Brønsted-Lowry definition of acids
What is acids are proton (hydrogen ion) donators
According to the enthalpy diagram, this chemical reaction is: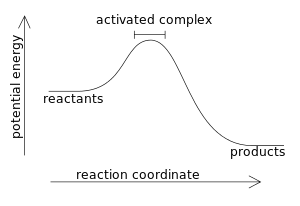
What is exothermic (release of energy)
What is the difference between fission and fusion?
What is fission - splitting nuclei. Fusion - joining nuclei.
Which of the following elements has the highest electronegativity?
Oxygen
Nitrogen
Carbon
Beryllium
What is Oxygen
The average kinetic energy of a substance is the definition of –
What is temperature
How many molecules of H2O are in 0.455 mols?
What is 2.74 x 1023 molecules
Vinegar is a homogeneous mixture of 10% acetic acid and 90% water. Which is
true regarding vinegar?
What is acetic acid is the solute and water is the solvent
Which of these chemicals would be an Arrhenius base?
H2SO4(aq) + 2NaOH(aq) → 2H2O(l) + Na2SO4(aq)
What is NaOH(aq)
A high temperature is related to –
what is molecules with high average kinetic energy.
List the three types of radiation from lowest energy to highest energy?
What is alpha, beta, gamma
Which of the following elements has the largest atomic radius?
Helium
Neon
Argon
Krypton
What is Krypton
What will cause a soluble solid to dissolve more quickly in water?
What is agitation(stirring), temperature, increase surface area
If you have 3.50 moles of ethane C2H6, how many moles of oxygen will be consumed(needed)?
2C2H6 + 7O2 --> 4CO2 + 6H2O
What is 12.3 moles O2
50 g of KNO 3 is dissolved at 60 °C, how much more KNO 3 can be dissolved at that temperature?
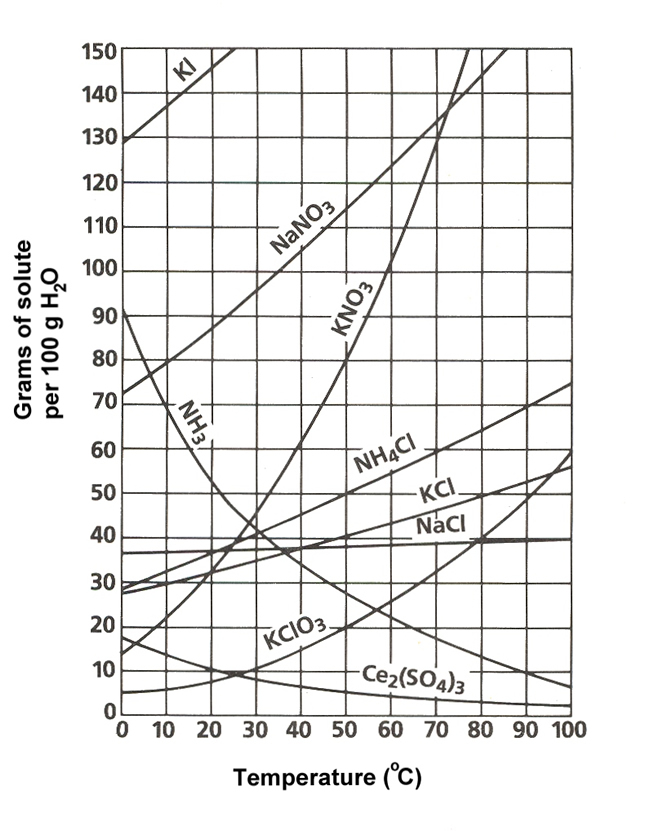
What is 40g
Which of these reactants would be an acid?
H2SO4(aq) + 2NaOH(aq) → 2H2O(l) + Na2SO4(aq)
What is H2SO4
Calculate the heat needed to raise 27.0 g of water from 10.0 °C to 90.0°C if the specific heat of water is 4.184 J/g• o C
Q= mCpΔT
What is +9040 J
Which form of decay is shown in the following nuclear equation?
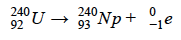
What is beta decay.
What family on the periodic table is typically not included in electronegativity trend?
What are Noble Gases
When phosphorous reacts with chlorine, it forms phosphorus trichloride (PCl3).The theoretical yield was calculated to be 32.50 g PCl3(l) . If the actual yield for this experiment was 23.89 grams what is the percent yield?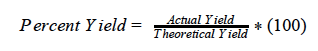
What is 73.51%
If you react 20.123 g of oxygen with silver, how many moles of silver oxide can
form?
4Ag(s) + O2(g) → 2Ag2O(s)
What is 1.2578 mols Ag2O
If a student dilutes 250 mL of 0.10 M lithium acetate solution to a volume of 750mL, what will the molarity of this solution be? Show Work:
M1V1 = M2V2
What is .033 M
What is the hydroxide [OH-] pOH value, if the hydronium ion [H3O+] concentration is 1 x 10-5?
What is pOH 9
What is type of chemical reaction is with a negative ΔH as shown in the example below?
CH4 + O2 → H2O + CO2
ΔH = -882 kJ/mol
What is exothermic
What is the complete nuclear equation for the alpha decay of the following isotope?
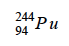
What is 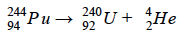
What direction does ionization increase?
What is Up and to the Right
Calculate the percent composition by mass of oxygen for the following substance:
Fe2(SO4)3
What is 48%