Define endothermic
A process that absorbs heat energy from its surroundings
Define condensation
The process by which gas particles lose energy and change into a liquid.
Why can gases expand to fill their containers?
Gas particles move freely and are far apart, filling any available space.
Is helium’s boiling point greater than or less than room temperature? How do you know?
Less than room temperature — helium is a gas at room temp, so it must boil far below it.
What does Charles’s Law describe, and what happens to gas particles when the temperature rises?
Charles’s Law: Volume ↑ as temperature ↑ (at constant pressure).
Higher temperature → faster-moving particles → more forceful collisions, causing the gas to expand.
Define Exothermic
A process that releases heat energy to its surroundings.
Define sublimation.
The direct change from solid to gas without passing through the liquid phase.
Why do solids have a fixed shape and volume?
Their particles are tightly packed and vibrate in place, maintaining a rigid structure.
If a substance has a very high melting point, what state is it probably in at room temperature?
Solid, because it needs lots of energy to melt.
What does Boyle’s Law describe, and how do particles behave when volume decreases?
Boyle’s Law: Pressure ↑ as volume ↓ (at constant temperature).
When the container’s volume shrinks, particles have less space and collide more often with the walls, increasing pressure
Example of an endothermic phase change and why.
Melting ice — heat is absorbed to break bonds between solid water molecules.
What happens on the particle level when a substance condenses?
Particles lose kinetic energy, move closer together, and form stronger attractions.
What does gas pressure measure, particle-wise?
The frequency and force of collisions of gas particles against container walls.
Predict a reasonable freezing point for simple syrup (sugar + water).
Below 0°C, since dissolved sugar lowers water’s freezing point.
What does Gay-Lussac’s Law describe, and how do particles cause pressure to change?
Gay-Lussac’s Law: Pressure ↑ as temperature ↑ (at constant volume).
As the temperature rises, particles move faster and hit the container walls more frequently and forcefully, raising pressure.
Example of an exothermic phase change and why.
Condensation — heat is released as gas particles slow down and form liquid attractions
Distinguish between the two ways a liquid can vaporize.
Evaporation occurs at the surface and below boiling point; boiling happens throughout the liquid at its boiling point.
What does temperature measure, particle-wise?
The average kinetic energy of the particles in a substance.
What is freezing point depression?
The lowering of a liquid’s freezing point due to dissolved solutes.
A gas sample’s volume doubles when its pressure is cut in half. Which law explains this, and how do particle collisions change?
Boyle’s Law — as pressure decreases, particles collide less often with the container walls because they have more space to move.
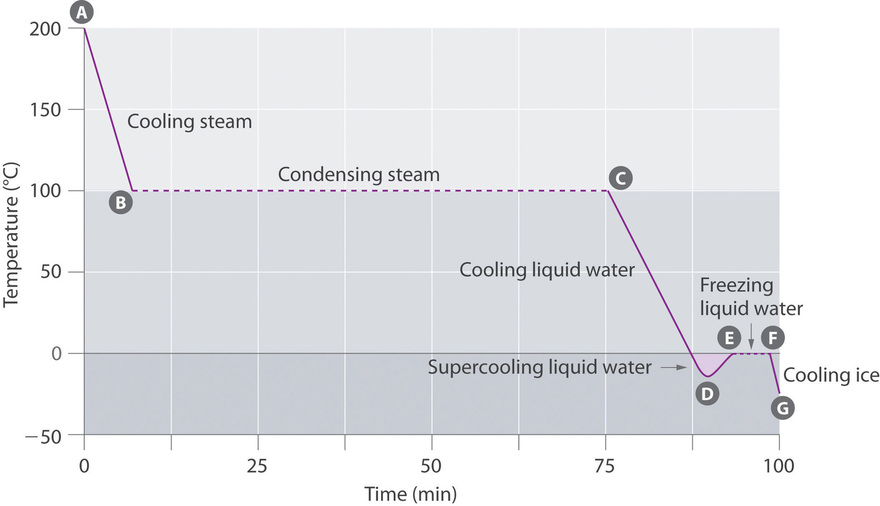
Sketch or describe a temperature-time graph for an exothermic phase change.
Why do water droplets form on the outside of a cold drink?
Water vapor in the air cools and condenses into liquid water on the cold surface.
How does particle behavior explain the relationship between temperature and pressure?
Higher temperature → faster-moving particles → more frequent and forceful collisions → higher pressure.
If the volume of a container of gas is doubled, the pressure of that gas will…?
Decrease by half (Boyle’s Law — pressure and volume are inversely related).
A helium balloon shrinks when placed in a freezer. Which gas law explains this, and what happens to the particles?
Charles’s Law — as temperature decreases, particles move more slowly and collide less forcefully, so the gas volume shrinks.