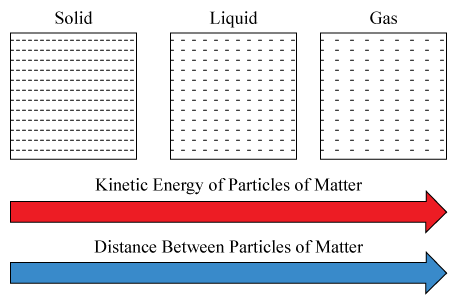
Do particles in the gas state have higher or lower kinetic energy than particles in the liquid state?
Higher
Liquid particles sliding past one another gives them the ability to __________.
Flow
Solids have a definite shape and volume. True or false?
True
What is 100°C in Kelvin?
373 K
Temp in K = Temp in °C +273
Which state of matter has an indefinite shape but a definite volume?
Liquids
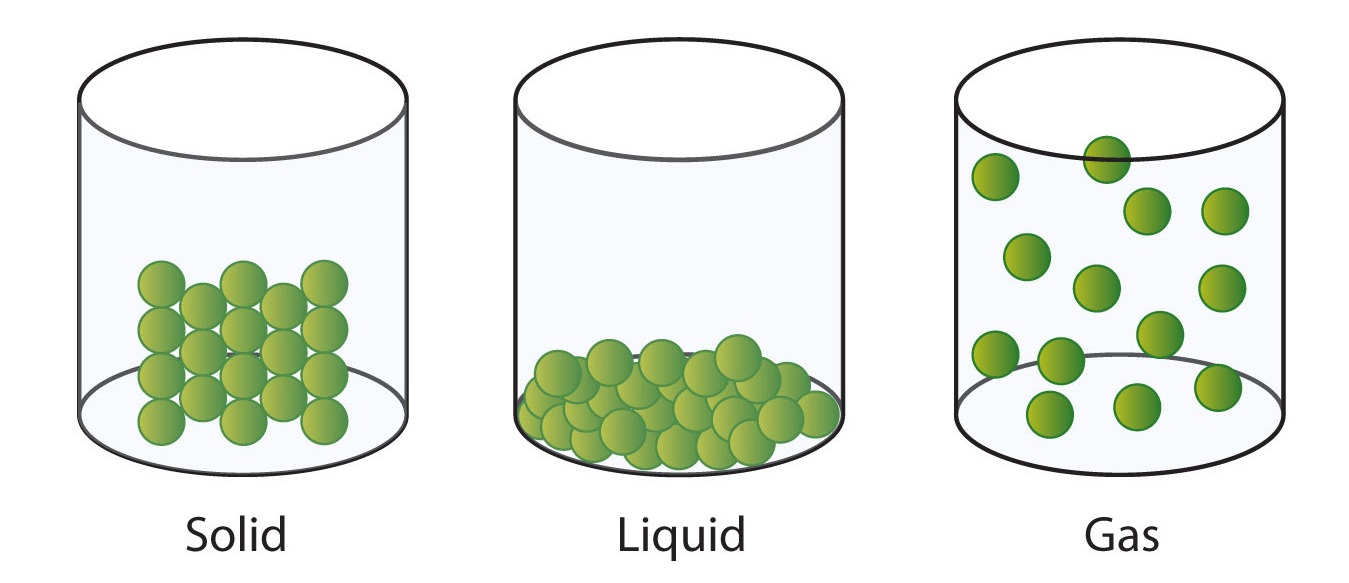
What causes gas pressure?
The collision of gas particles with each other.

Describe the strength of the intermolecular forces within liquids.
Stronger than gases but weaker than solids.

What is the freezing point of a substance?
The temperature at which liquid changes to solid.

What belongs on the x-axis of a phase diagram?
What belongs on the y-axis?
Temperature on the x-axis.
Pressure on the y-axis.
Which state of matter has the strongest intermolecular forces?
Solids
Gases have the strongest intermolecular forces. True or False?
False. Solids have the strongest IM forces.
If you increase the temperature of a liquid, the rate of evaporation of that liquid will also increase. True or false?
True
Define sublimation.
A change in state from solid directly to gas.
:max_bytes(150000):strip_icc()/sublimation-of-dry-ice-co2-solid-co2-changes-directly-from-solid-to-gas-128108785-5768263e5f9b58346ad1d386.jpg)
Which state of matter is the most dense?
Solid
Liquid A has much weaker intermolecular forces than Liquid B. Which would you expect to evaporate faster?
Liquid A

What is standard pressure in kilopascals (kPa)?
101.3 kPa
Is evaporation a cooling or heating process?
Cooling

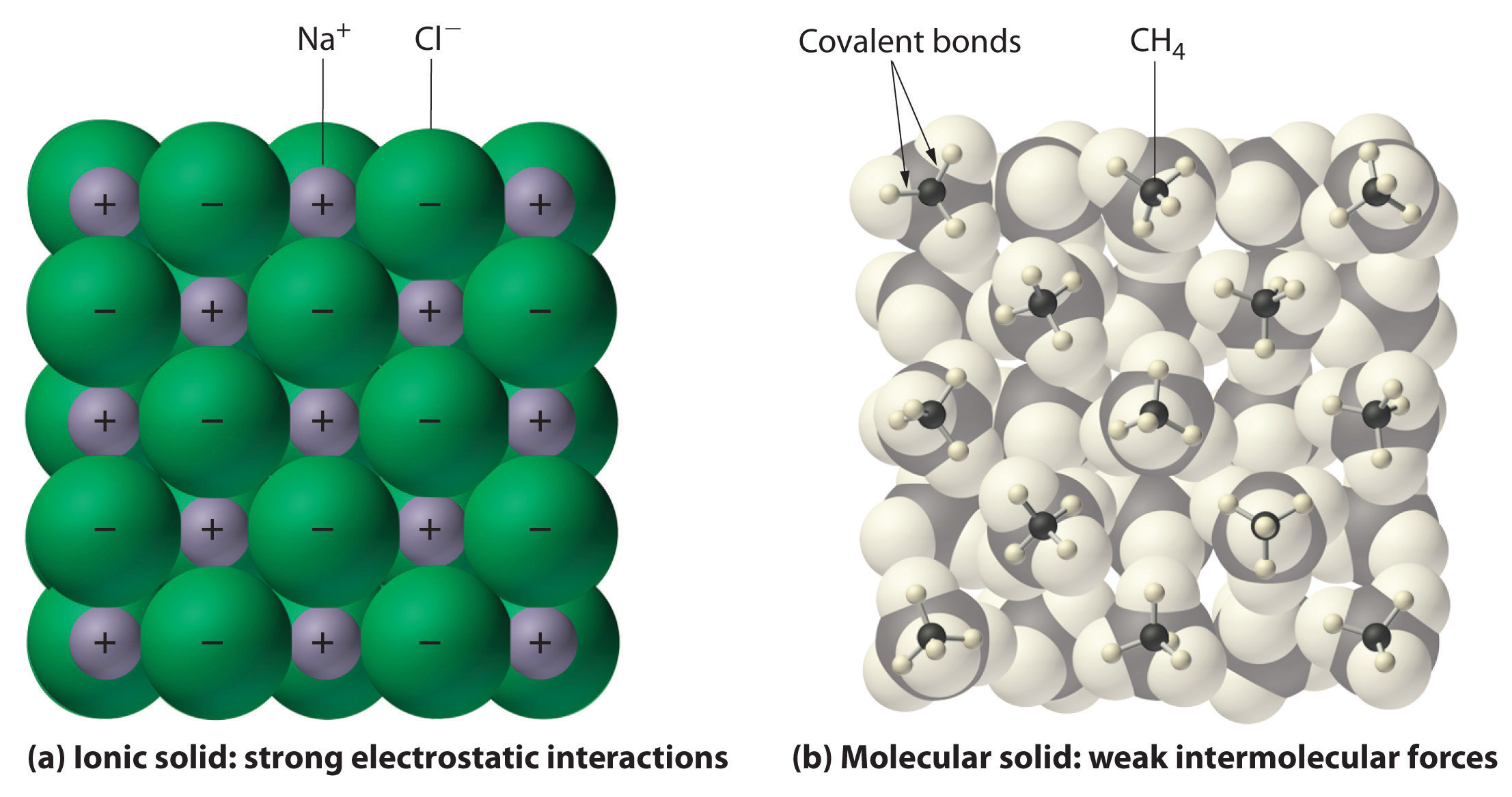
Do ionic or molecular solids have stronger intermolecular forces?
Ionic solids
What is the boiling point of water?
100 °C
The energy of motion is known as....
Kinetic energy
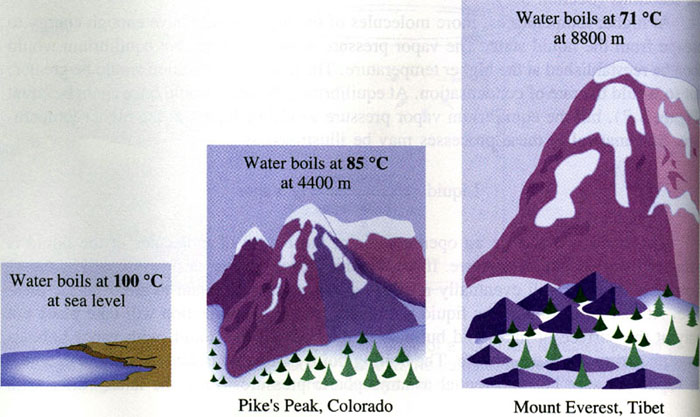
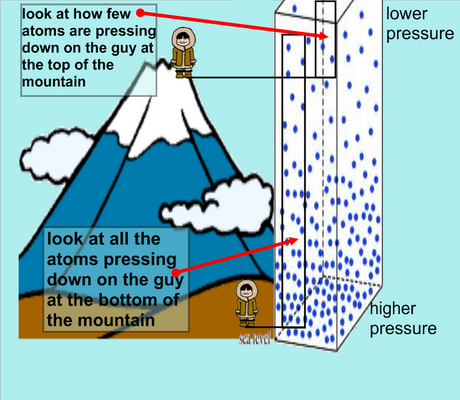
As altitude increases, does atmospheric pressure increase or decrease?
Decrease.
Define the normal boiling point of a liquid.
The temperature at which the vapor pressure of the liquid is equal to standard pressure.
VP = 1 atm
The melting points of molecular solids tend to be much higher than ionic solids. True or false?
False. Ionic solids tend to have higher melting points because of the strong attraction between particles.

1 atm = 101.3 kPa = 760 mmHg
Convert 5.01 atm to kPa.
508 kPa
Is the boiling point of water higher or lower on top of a mountain than it is at sea level (i.e., standard pressure)?
Lower than at sea level