The four states of matter.
What solids, liquids, gases, and plasma
Energy of motion.
What is Kinetic energy
Evaporation is a change from this starting phase to this ending phase.
The phase pictured here.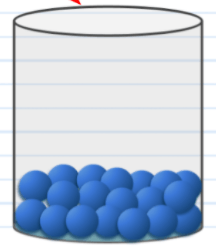
What is liquid
As temperature decreases, molecules move...
What is slower
The state of matter that has a definite shape and definite volume.
What is solid
A measure of the average velocity of the particles in a substance.
What is temperature
This phase change is a change from a liquid to a solid.
What is freezing
This phase change is pictured here.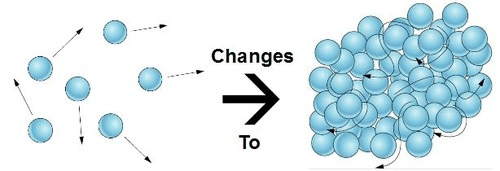
What is condensation
The boiling point of water.
What is 100 degrees C
The only state of matter with charged particles.
What is plasma
Energy is ________________ when changing states from a liquid to a solid.
When melting occurs, particles will move...
What is faster
The state of matter in this picture moves in this way.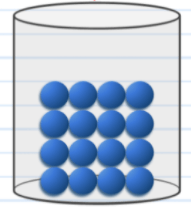
What is by vibrating
0 degrees C is the temperature at which these two phase changes occur.
What are Melting and Freezing
The particles of this state of matter are in constant motion.
What is solids, liquids, gases, and plasma
These two phase changes do not involve liquids.
What are deposition and sublimation
Provide an example of deposition.
Frost forming on car/leaves, snow.
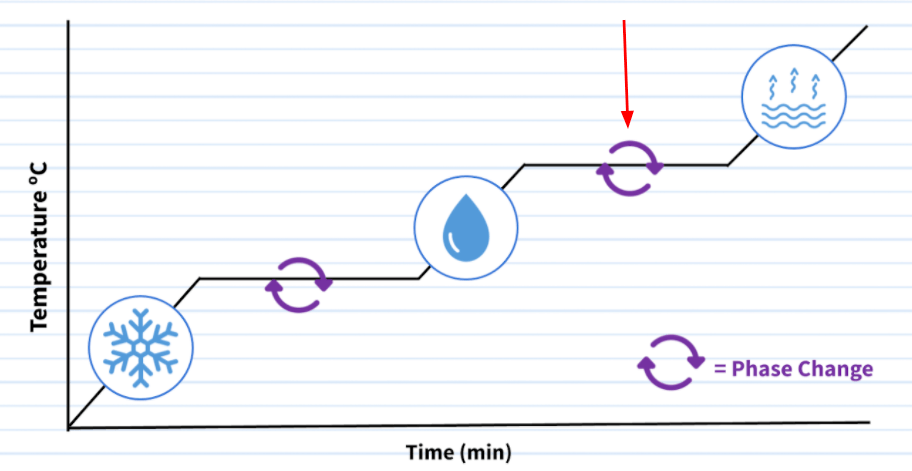 The phase changes that being pointed to by the red arrow.
The phase changes that being pointed to by the red arrow.
What is boiling or vaporization
These states of matter take the shape of their container.
What are liquids and gases
This temperature is when absolute zero occurs.
What is 0 Kelvin/-273 C.
This is the reason that air is "dry" in the winter.
Because the weather is cold, particle motion decreases therefore particles of water are not in the gaseous phase. The particles of water are liquid or solid, not in the air. Therefore the air is dry.
During a phase change, temperature...
What is does not change/remains the same
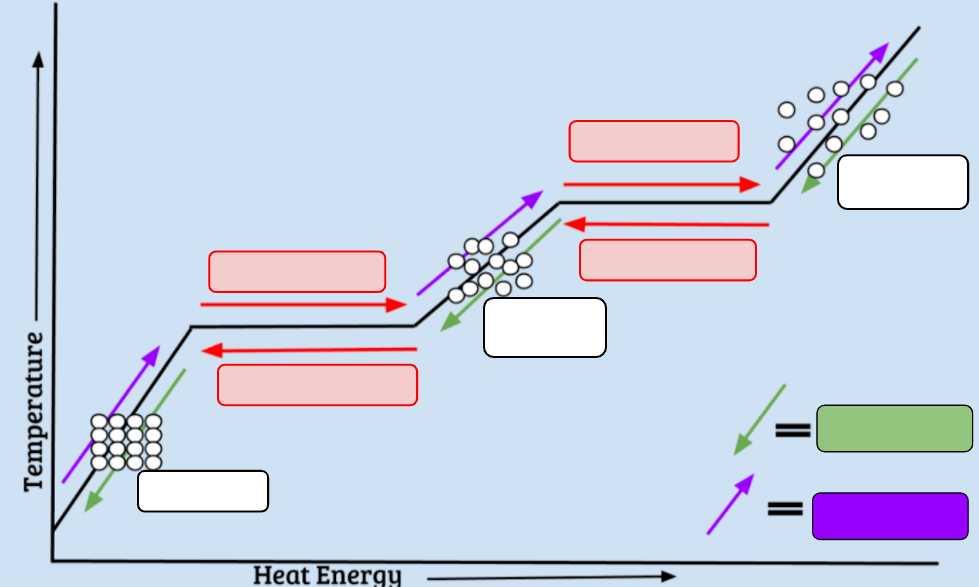 The green arrows on the diagram indicate that these phases are going through this type of change
The green arrows on the diagram indicate that these phases are going through this type of change
What is exothermic
The reason that pipes burst when water freezes.
What is ice is less dense than liquid water. It expands when froze, causing pipes to burst